Monoclone antibody of H5 subtype avian influenza virus haemagglutinin protein, or its combination liveness fragment and uses thereof
A monoclonal antibody and avian influenza virus technology, applied in the direction of antibodies, viral peptides, anti-animal/human immunoglobulin, etc., can solve the problems that cannot meet the needs of clinical testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] Embodiment 1: Preparation of anti-H5 subtype avian influenza virus HA gene monoclonal antibody
[0222] Antigen preparation:
[0223] The virus strain Ck / HK / Yu22 / 02 (H5N1) (referred to as Yu22) was used to inoculate 9-day-old fertilized chicken embryos, and after incubation at 30□ for 2 days, the chicken embryo liquid was collected to obtain the amplified Yu22 virus. Collect the live virus, inactivate it with 0.03% formalin formalin at 4°C, and detect the inactivated virus by HA to determine the titer of the inactivated virus solution (note: refer to the WHO operation for the specific methods of HA titer determination and HI detection guideline, we chose HA=1024, which was provided by the Department of Microbiology, University of Hong Kong).
[0224] mouse
[0225] Six-week-old female Balb / c mice were purchased from the Anticancer Center of Xiamen University and bred there for experiments.
[0226] Hybridoma preparation:
[0227] We use standard in vivo immunization...
Embodiment 2
[0242] Embodiment 2: Assembly of H5 subtype influenza virus HA antigen detection kit (enzyme-linked immunoassay, ELISA)
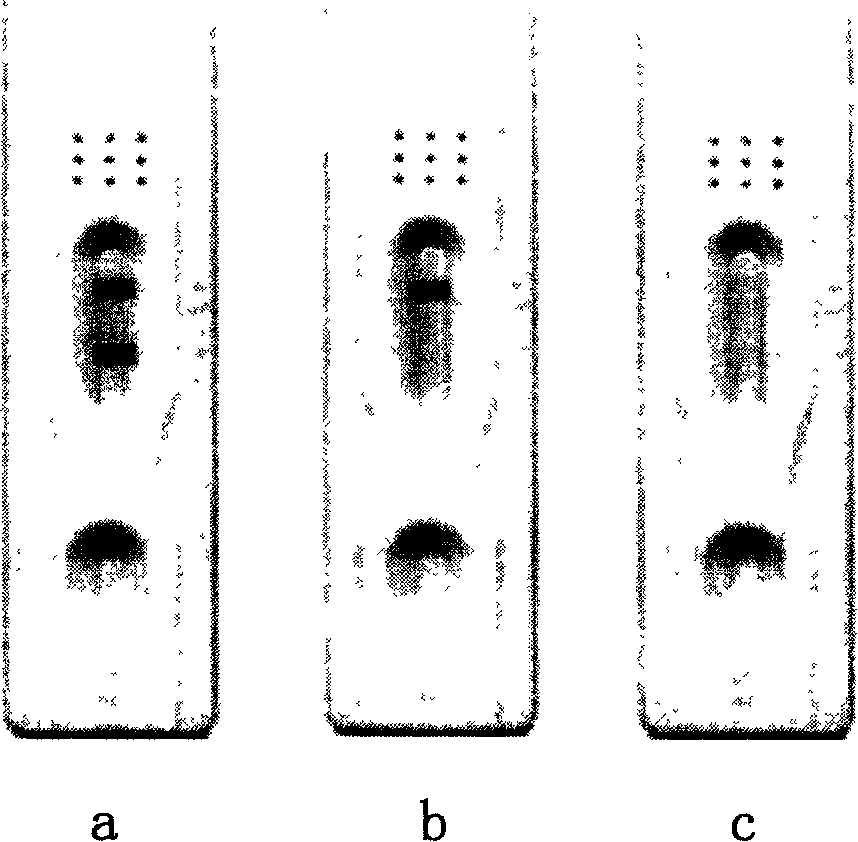
[0243] This kit uses the double-antibody sandwich method to detect the HA antigen of the H5 subtype influenza virus in the specimen. First, the monoclonal antibody against H5 influenza virus HA gene is pre-coated on the polyethylene microwell strip of the kit. When the cleaved H5 influenza virus HA antigen is added to the microwell, the pre-coated monoclonal antibody It can be captured, and then the enzyme-labeled monoclonal antibody added can also bind to it, and then the result can be judged by the degree of color development of the enzyme-catalyzed substrate. When the specimen does not contain influenza virus antigen or H5 influenza virus, the substrate will not develop color. Detectable specimens include excreta, oral and nasal secretions, whole virus or lysed virus cultured in chicken embryos, etc.
[0244] Preparation of microtiter plate:
[0245] ...
Embodiment 3
[0282] Embodiment 3: Assembly of H5 subtype influenza virus anti-HA antibody detection kit (enzyme-linked immunoassay, ELISA)
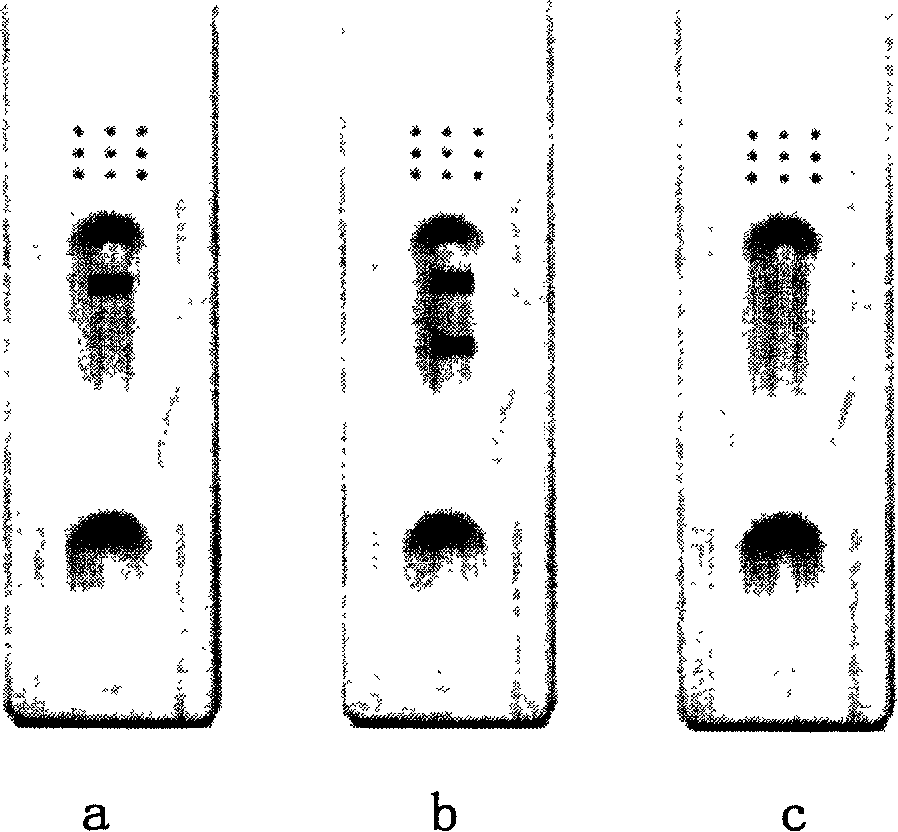
[0283] This kit uses a competitive method to detect H5 influenza virus HA antigen-specific antibodies in serum samples. Firstly, the monoclonal antibody against H5 subtype influenza virus HA is coated once on the microwell strip in the kit, and the recombinant expression antigen of H5 subtype influenza virus HA gene is coated twice. After adding the serum sample and the enzyme-labeled monoclonal antibody, the specific antibody in the sample competes with the enzyme-labeled monoclonal antibody to bind to the antigen on the enzyme-labeled plate. If the added serum sample can significantly inhibit the binding of the enzyme-labeled monoclonal antibody to the antigen , it indicates that the specimen contains specific antibodies to influenza virus H5 type HA antigen. When the specimen does not contain influenza virus antibody or H5 influenza virus antibody...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com