Avian adenovirus 4-type strain, vaccine composition and application of strain
A technology of vaccine composition and poultry adenovirus, applied in vaccines, viruses, antiviral agents, etc., to achieve broad application prospects, reduce economic losses, good specificity and immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Isolation and identification of avian adenovirus type 4 HNJZ strain
[0030] 1.1 Isolation of virus
[0031] Take the liver of chicken suspected of chicken hepatitis-pericardial effusion syndrome in Henan area, cut it into pieces with sterile scissors, add sterile PBS (pH7. Grind completely, collect the homogenate after grinding, transfer it to a sterile centrifuge tube, centrifuge at 10000g for 10min, filter the supernatant after centrifugation with a 0.22μm filter membrane, and use the filtrate to inoculate 11-day-old SPF chicken embryos Monolayer of primary chicken embryo liver cells at 37 °C 5% CO 2 After culturing, the cells were observed for lesions every day, and the cells showed typical adenovirus grape cluster lesions. Harvest the virus when the cell lesion reaches 90%. After repeated freezing and thawing three times, centrifuge at 10,000 g for 10 minutes.
[0032] 1.2 Virus identification
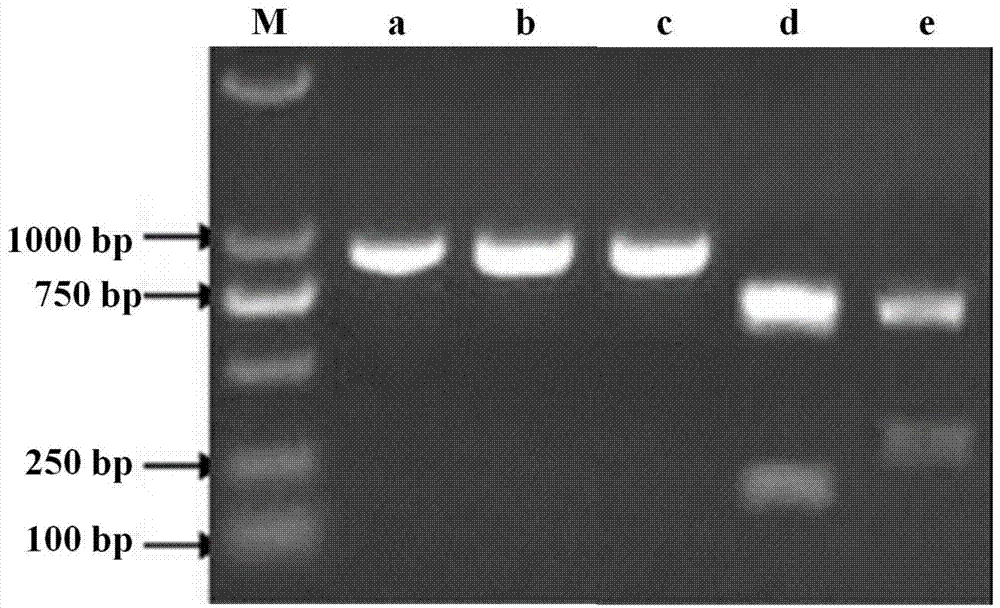
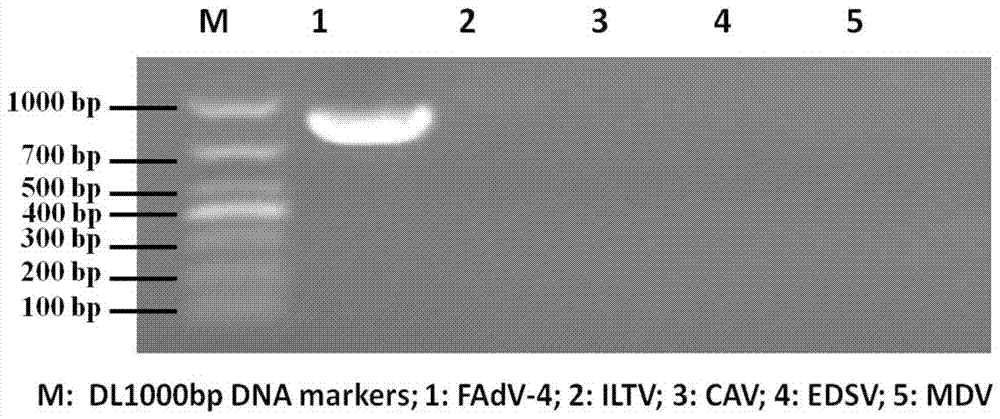
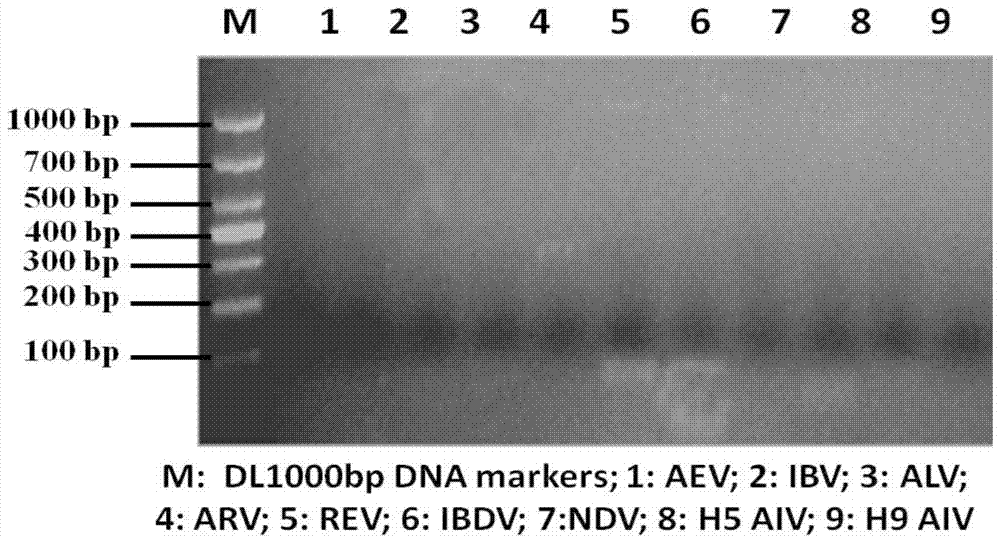
[0033] Primers were designed according to the Hexon gen...
Embodiment 2
[0045] Example 2: Sequence determination and analysis of avian adenovirus type 4 HNJZ strain
[0046] 2.1 Sequence determination of avian adenovirus type 4 HNJZ strain
[0047] Utilize the QIAamp DNA Blood Mini kit of Qiagen Company to extract its viral DNA from the virus isolated from Example 1 step 1.1 as a template, according to the genome sequence of the avian adenovirus type 4 ON1 strain (GenBank accession number GU188428) published in GenBank 26 pairs of specific primers (Table 1) were designed to perform PCR amplification reaction on the genome of avian adenovirus type 4 HNJZ strain. PCR amplification reaction system (50 μL) is: 5×PCR Buffer 10uL, template 10ng, 10mM deoxyribonucleoside triphosphate (dNTP) 1μl, upstream and downstream primers (25μM) each 1μl, high-fidelity thermostable DNA polymerase 1 unit , ddH 2 O make up. The PCR amplification reaction conditions were: pre-denaturation at 98°C for 2 min, 1 cycle of amplification; denaturation at 98°C for 10 s, an...
Embodiment 3
[0064] Embodiment 3: avian adenovirus type 4 HNJZ strain virus titer TCID 50 Determination of
[0065] Take 9-day-old SPF chicken embryos, after the eggshells are sterilized by 75% alcohol, open the eggshells from the air chamber side, take out the embryo body, remove the head and limbs with sterile ophthalmic scissors, take the liver, cut it into pieces, and wash it with sterile PBS Three times, add 0.25% trypsin, digest in a water bath at 37°C for 10 minutes; then add medium to blow the digested tissue into single cells, centrifuge at 200g for 5 minutes, and collect the cells; reweight in DMEM medium containing 10% fetal bovine serum Suspend the cells to get the hepatocyte suspension, count the hepatocyte suspension, and then count the number according to 1×10 5 The amount of inoculum of cells / well inoculated the hepatocyte suspension into a 96-well cell culture plate; the virus liquid obtained in 1.1 separation in Example 1 was diluted 10 times, and 10 -5 、10 -6 、10 -7 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com