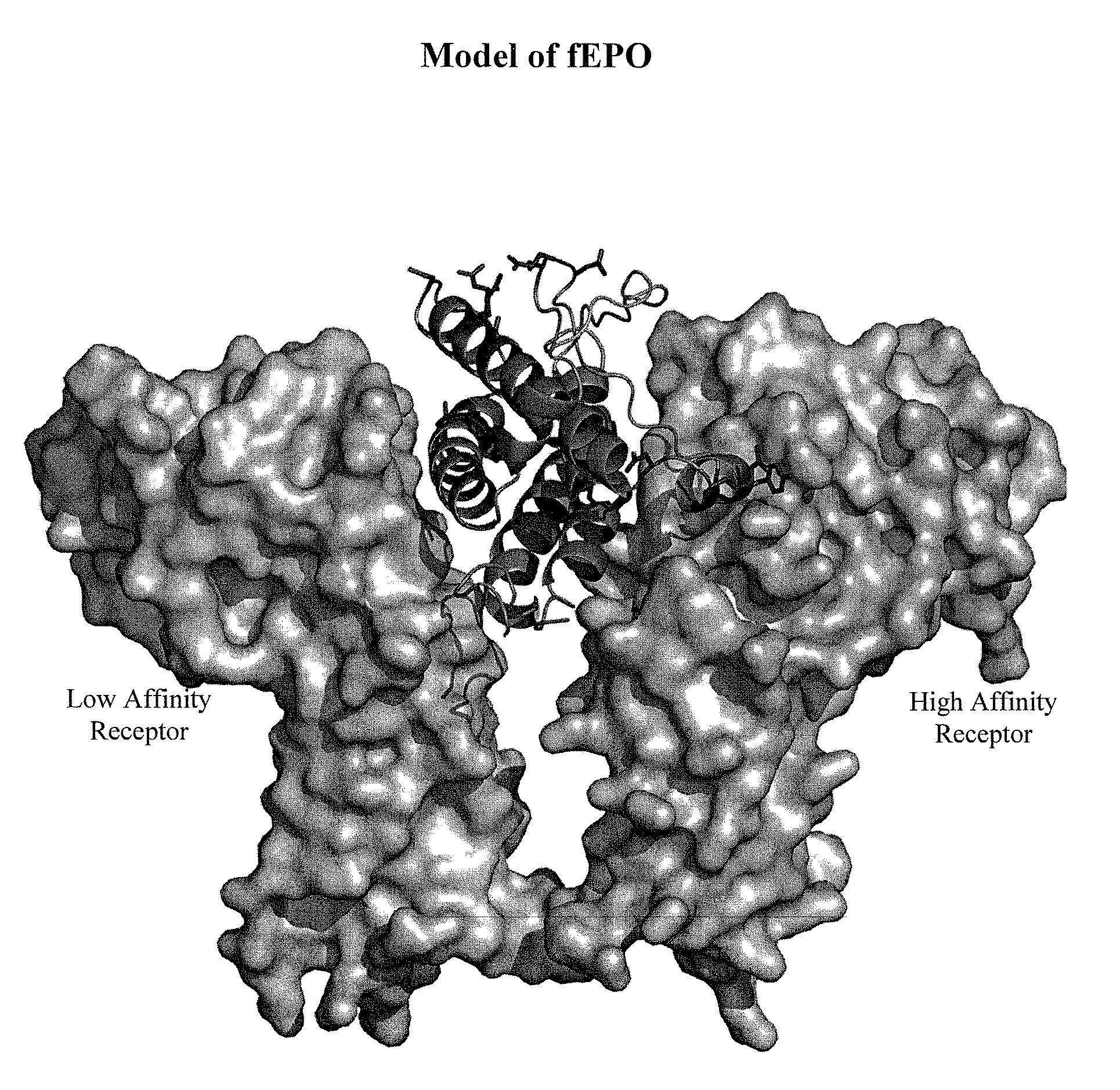
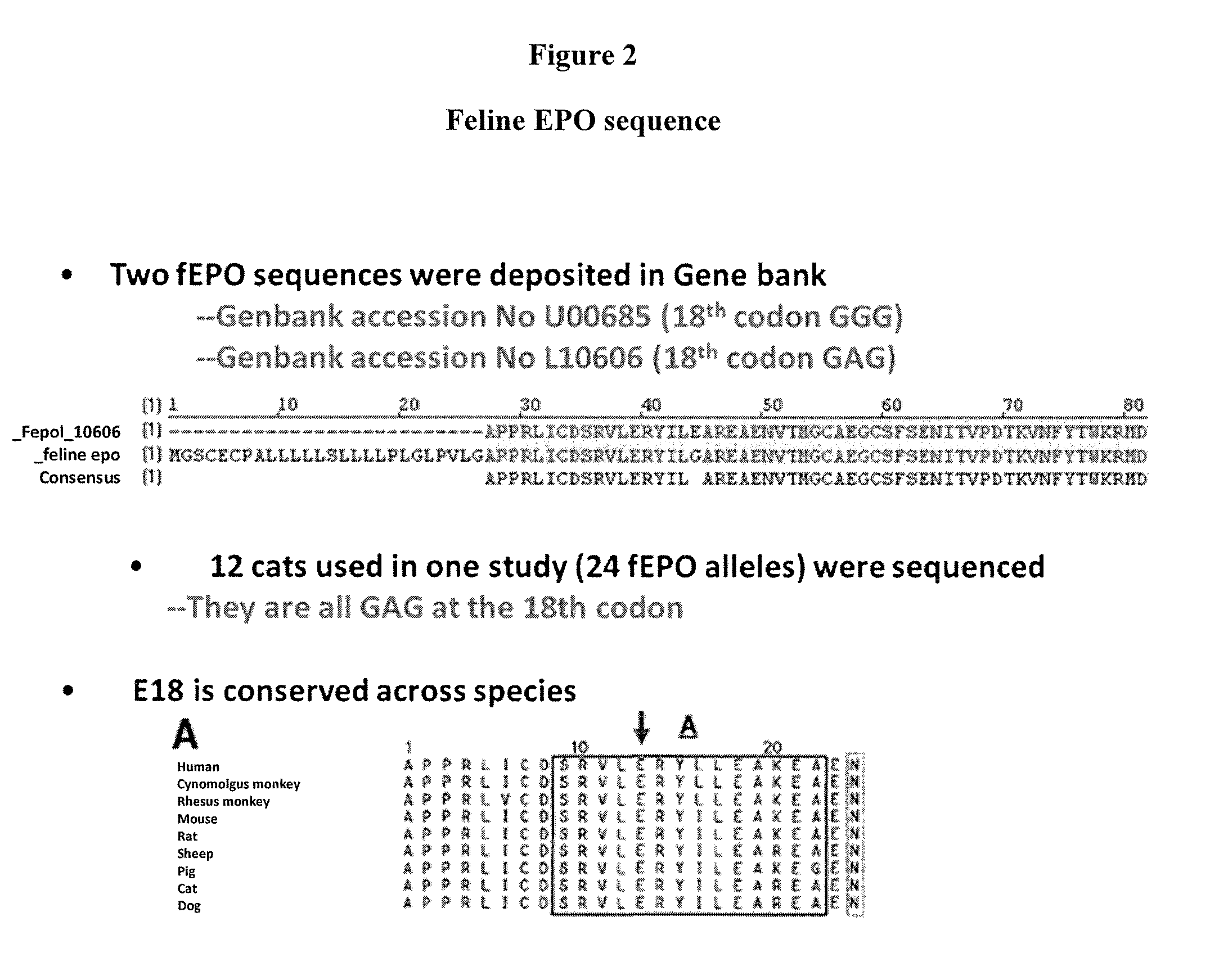
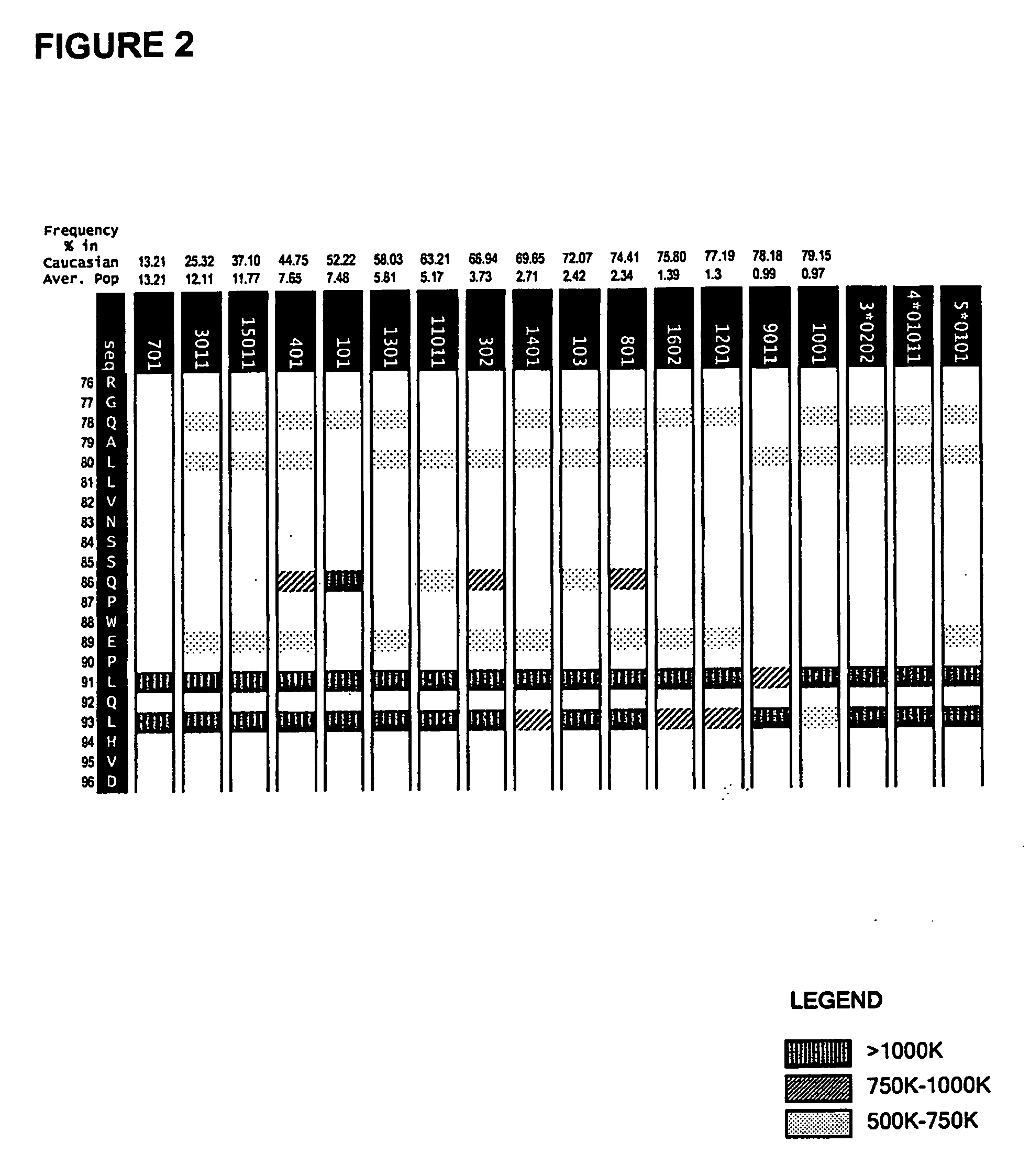
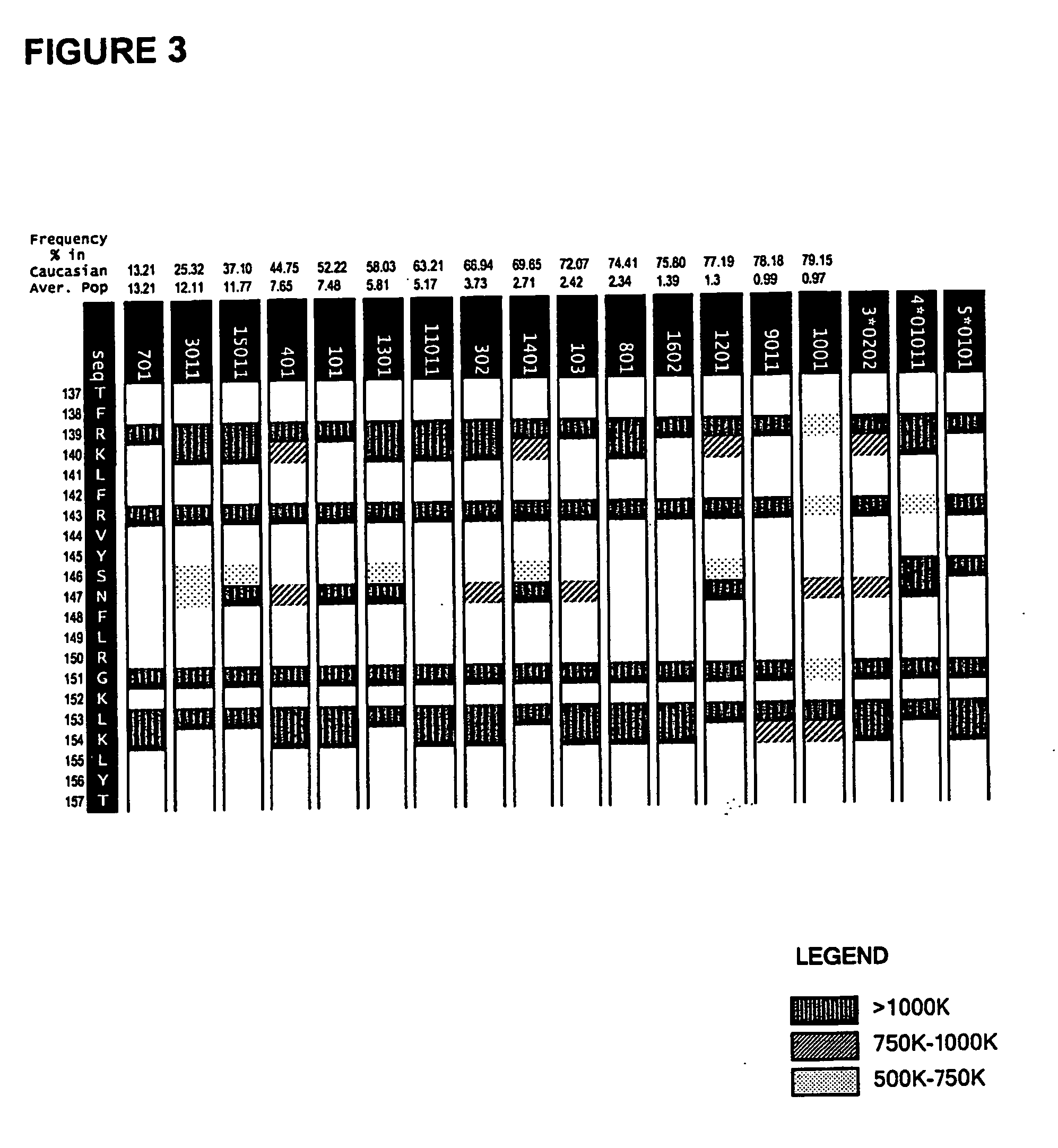
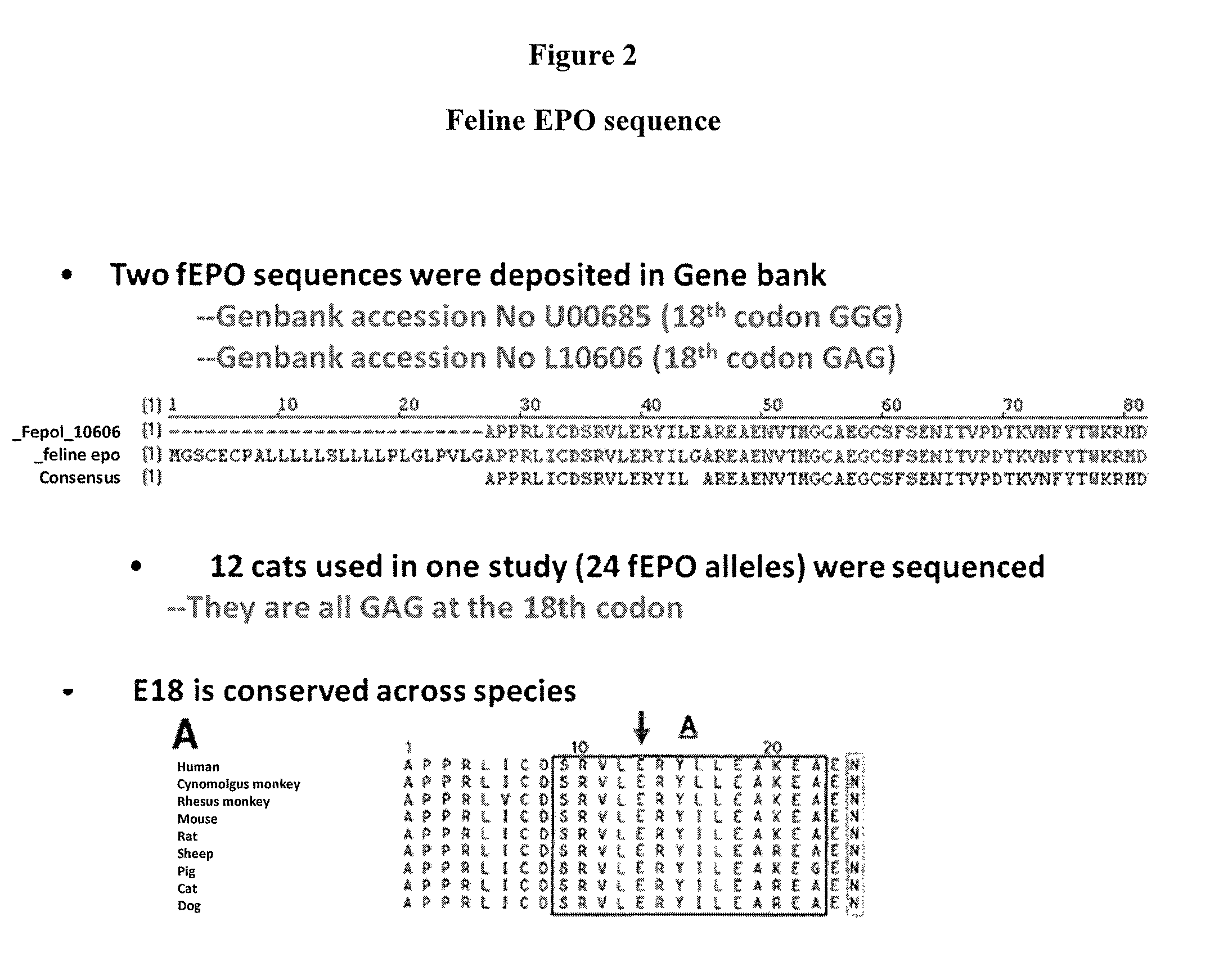
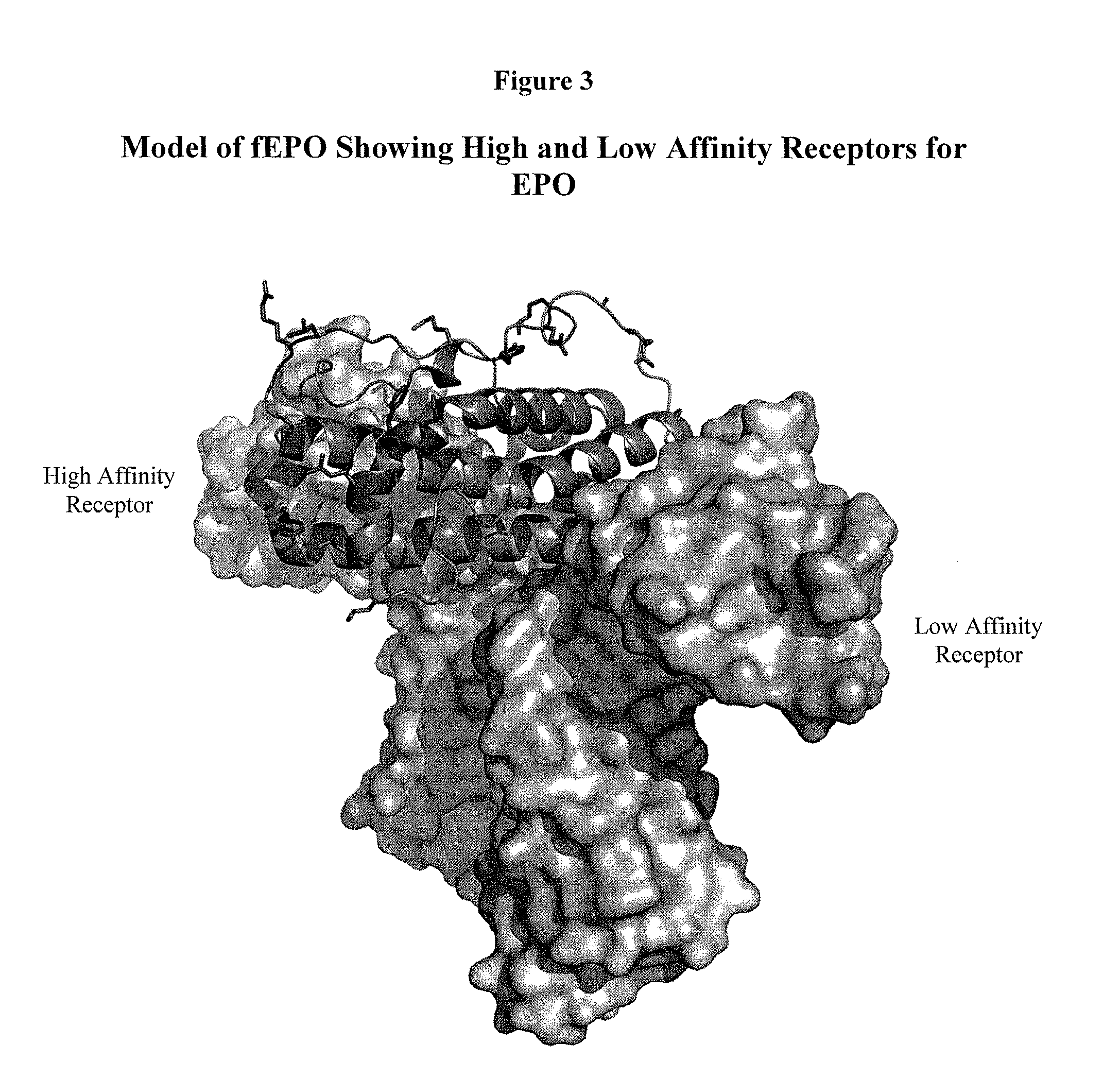
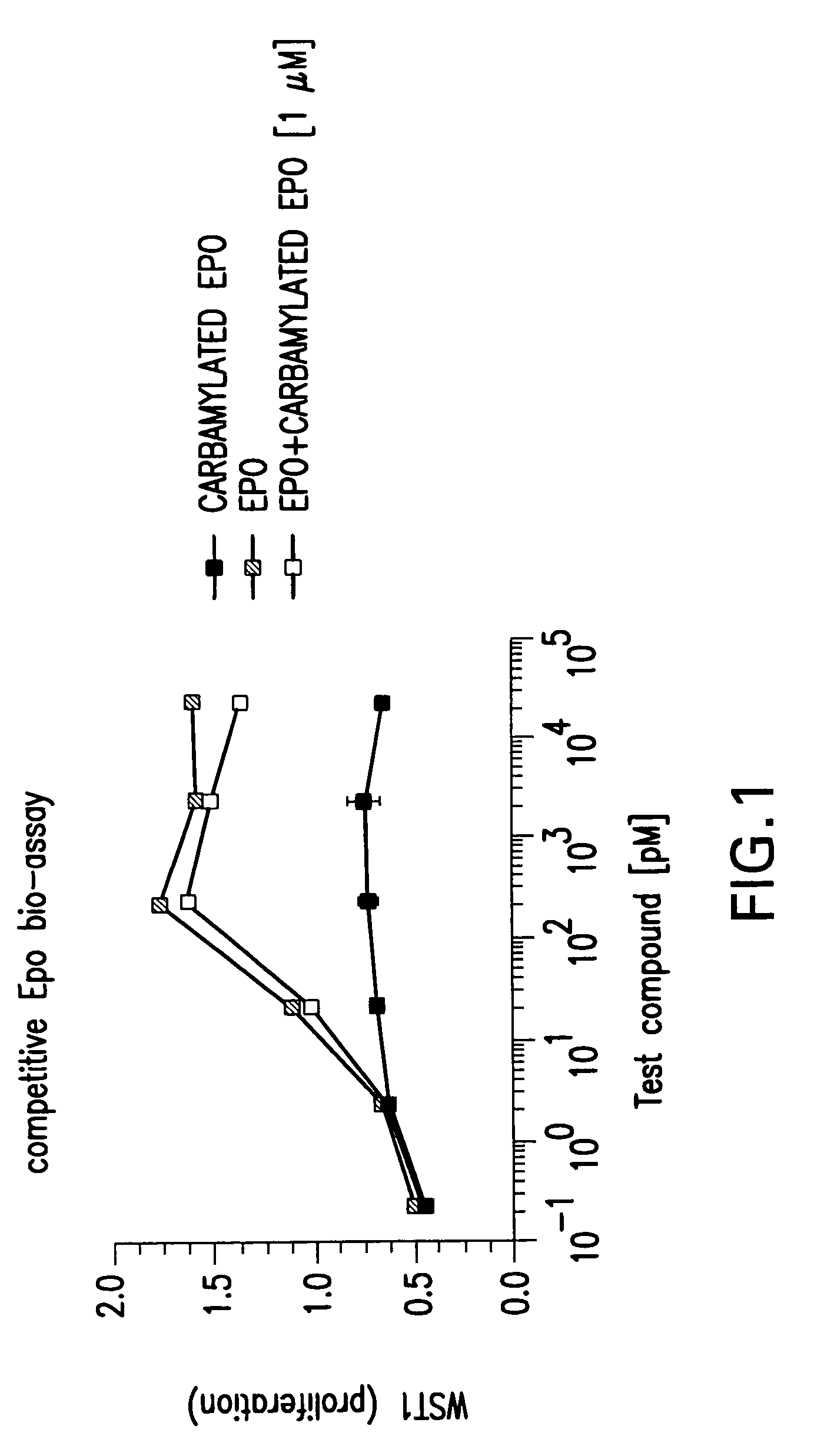
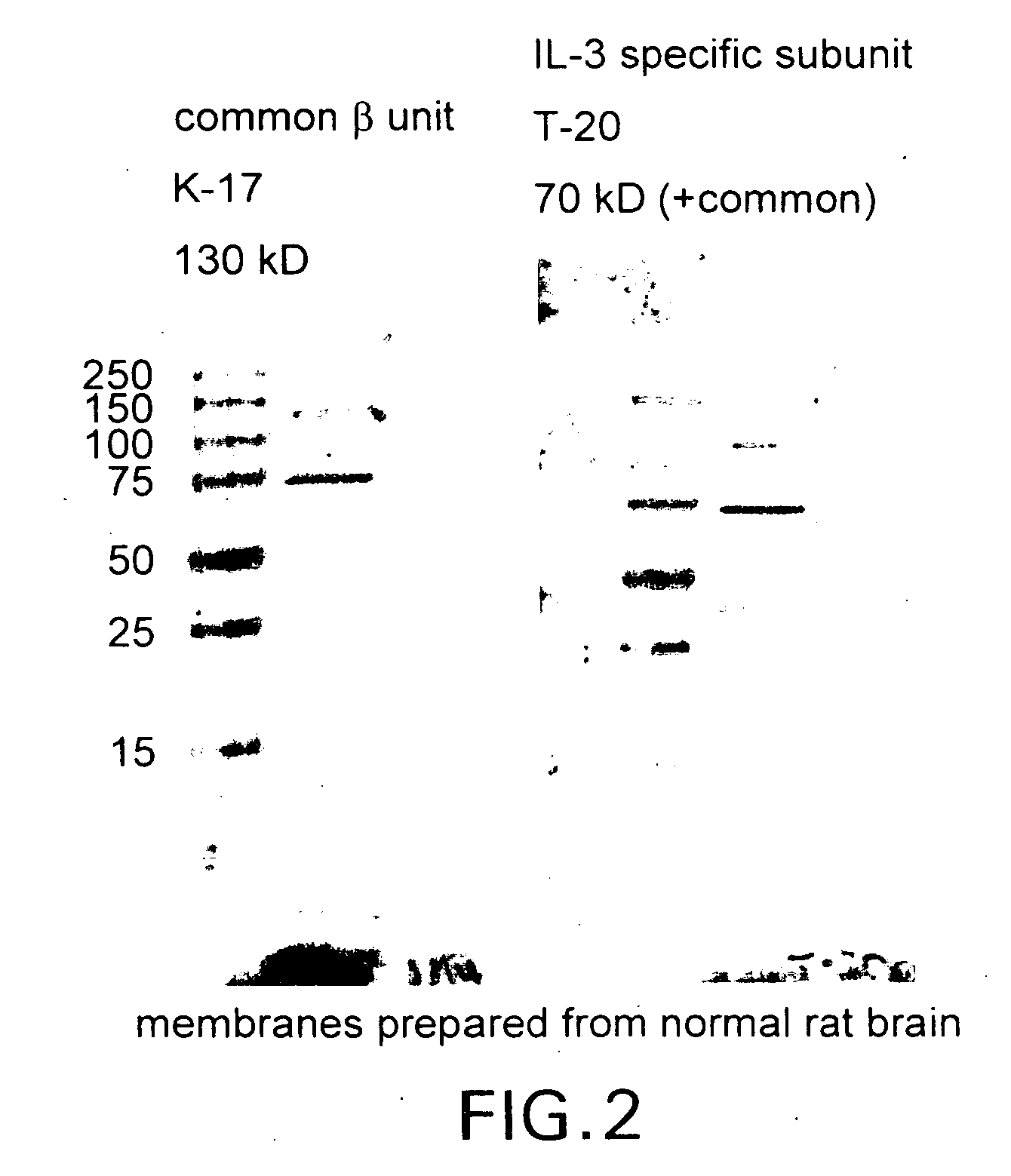
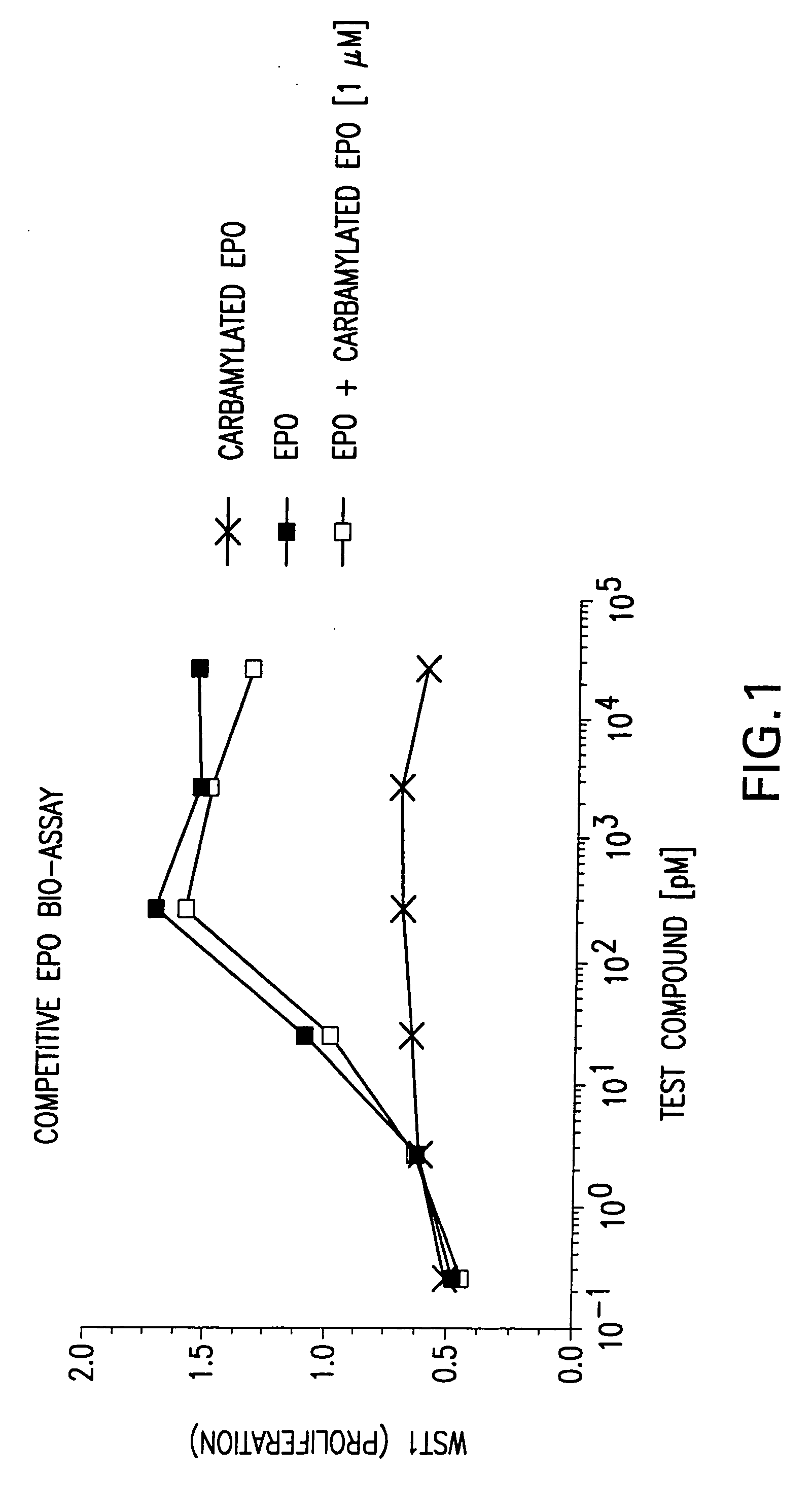
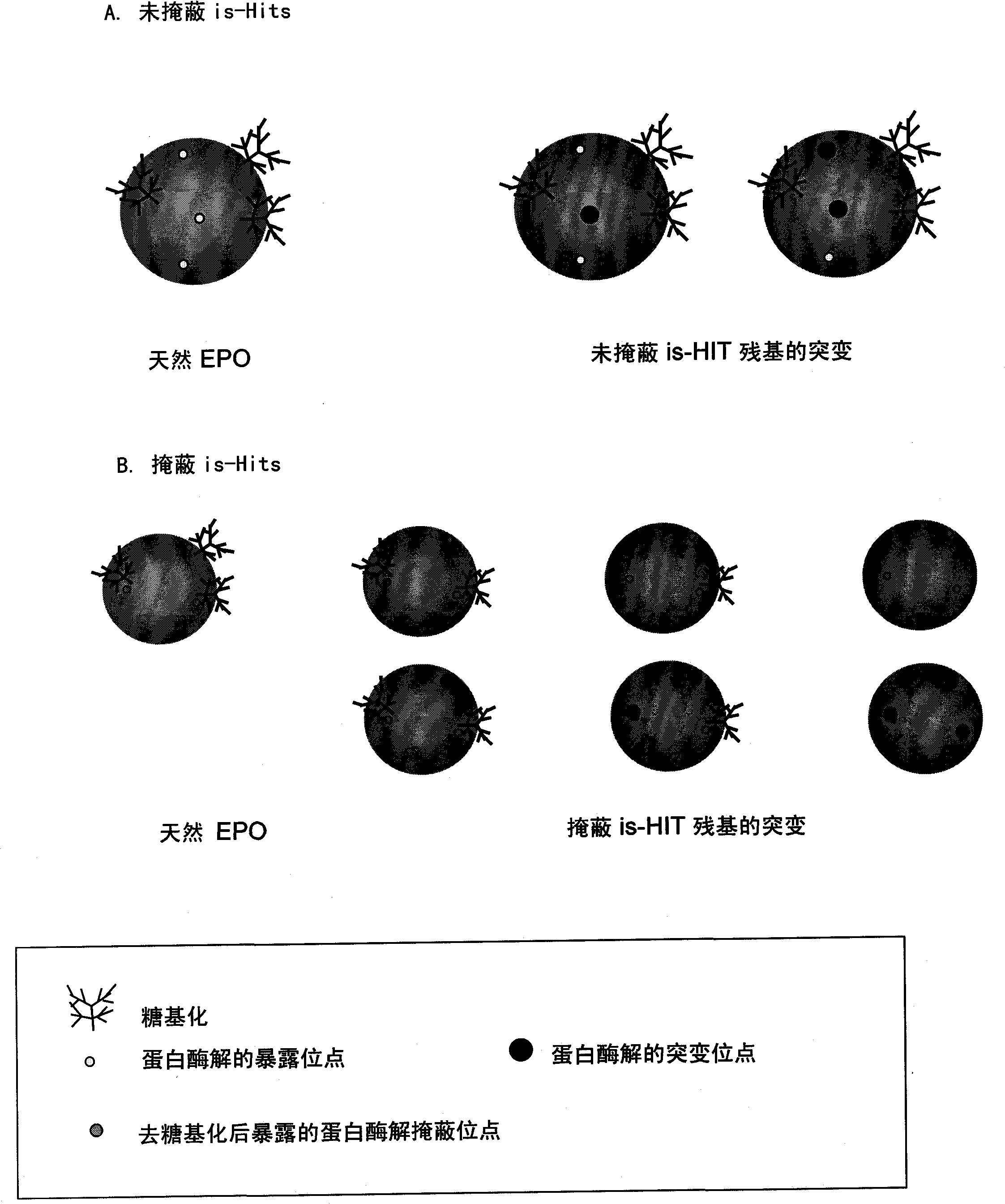
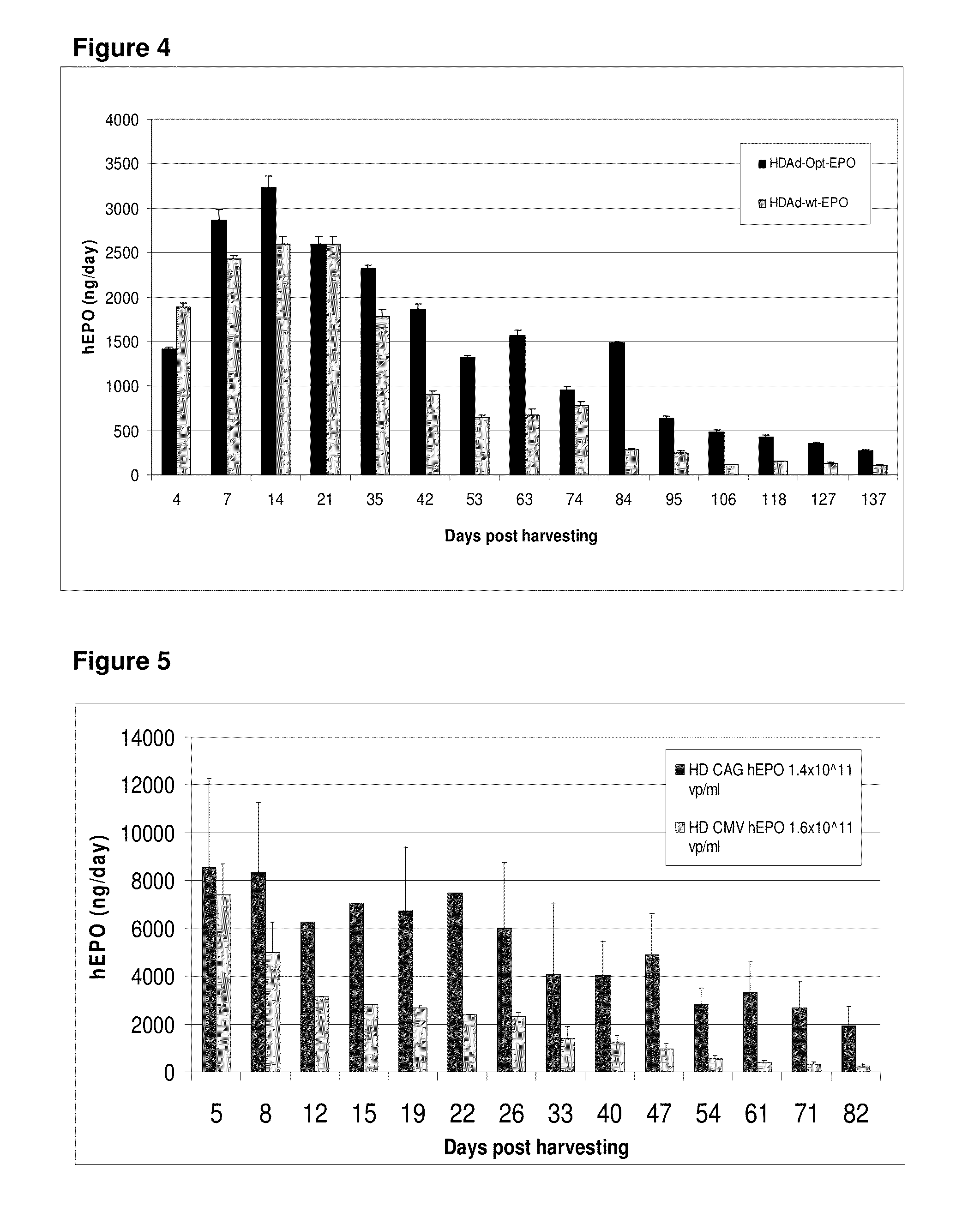
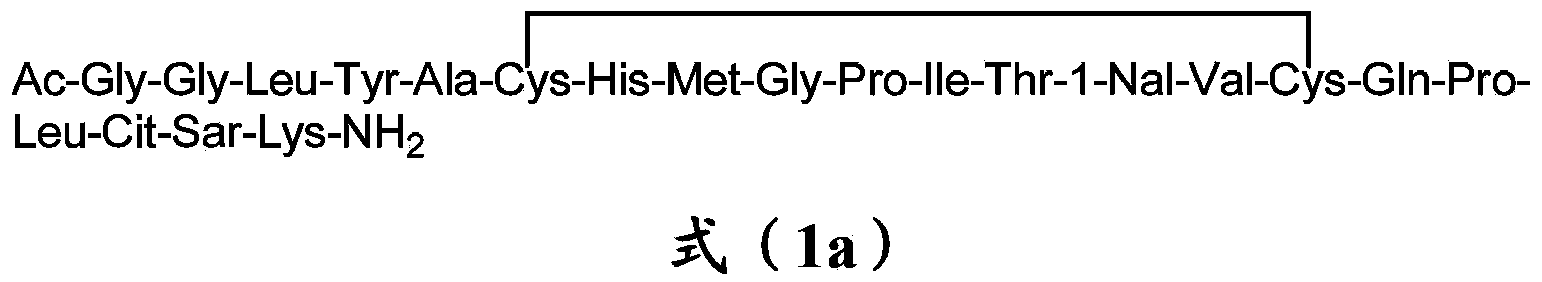Patents
Literature
69 results about "EPO - Erythropoietin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
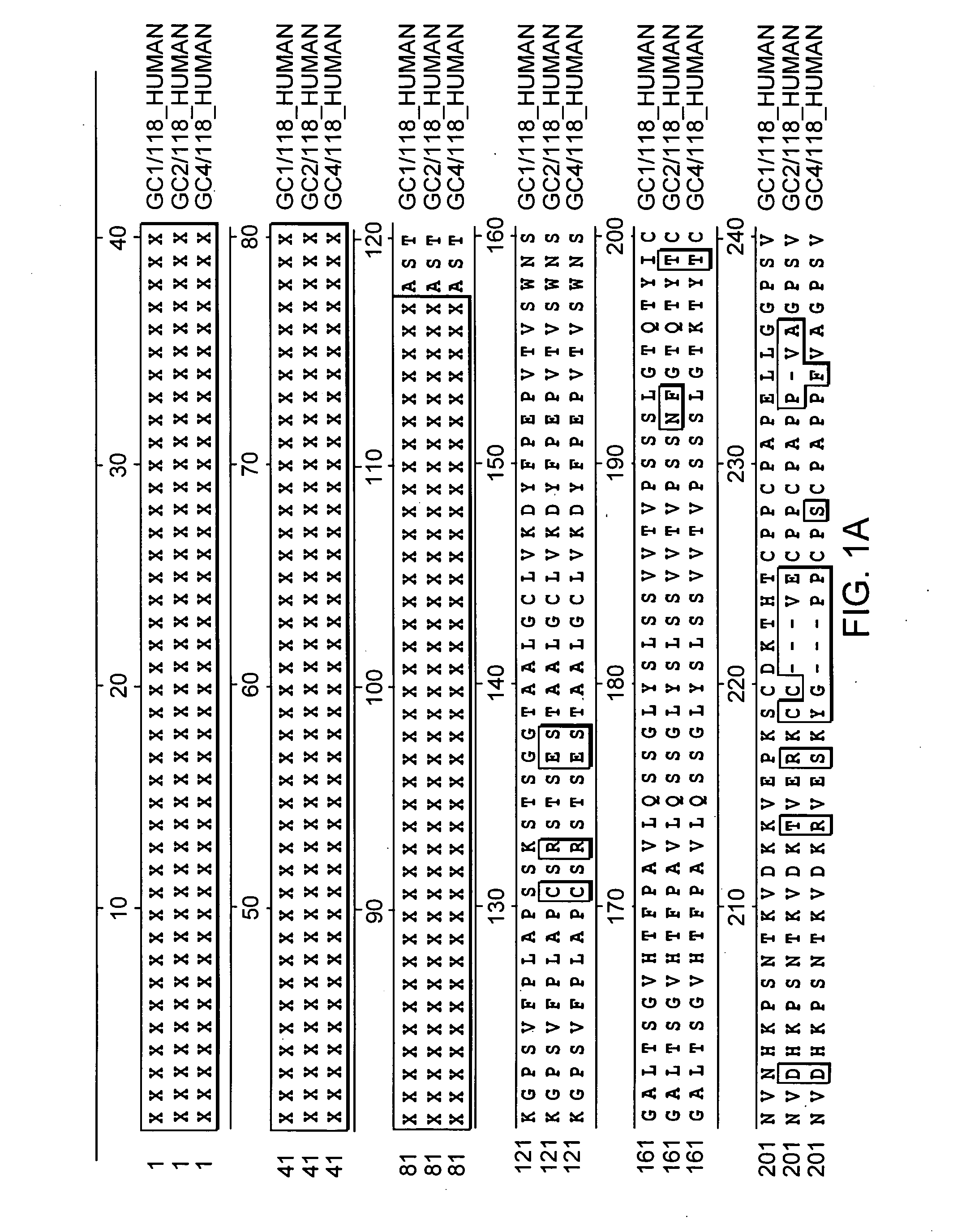
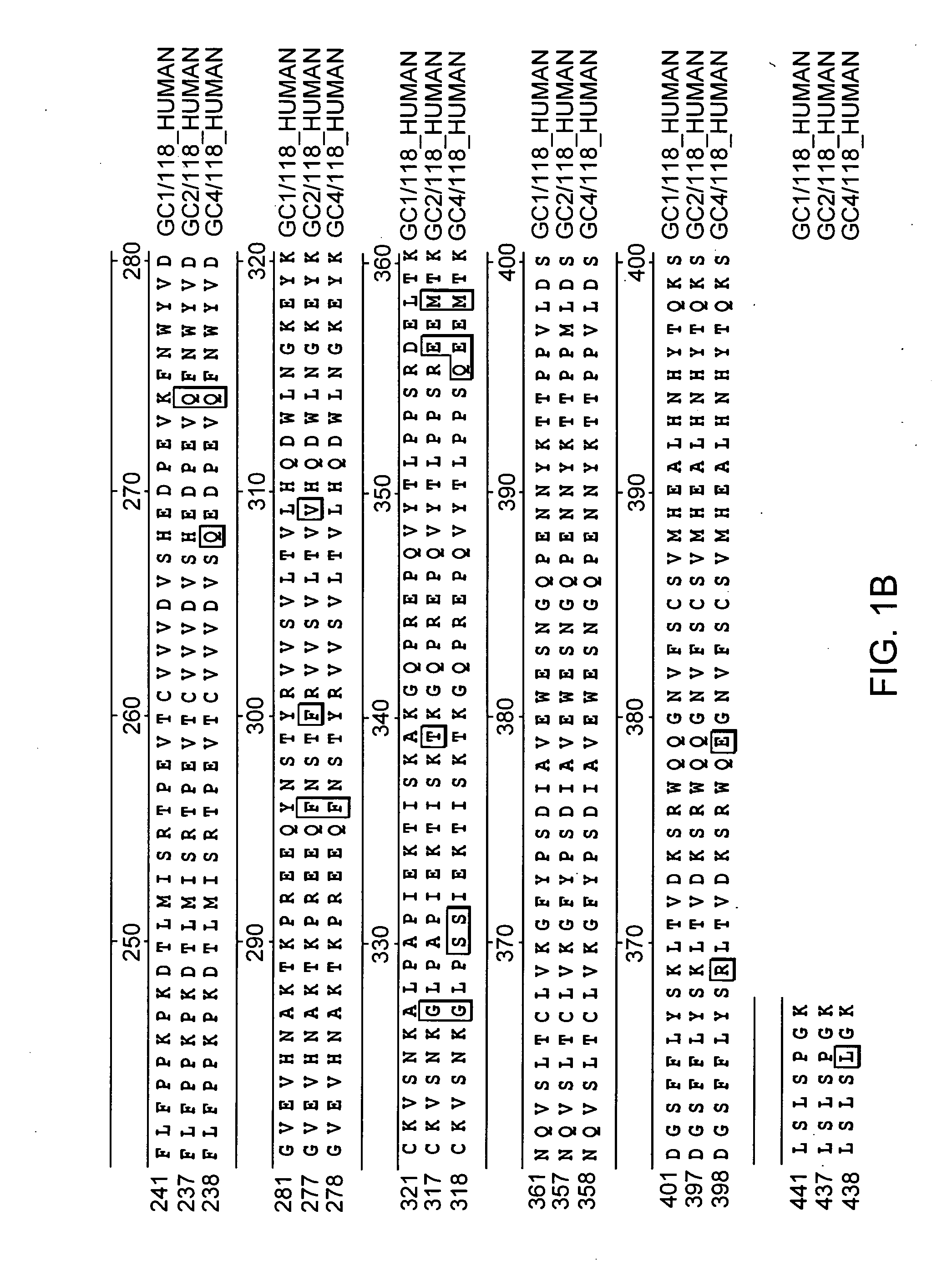
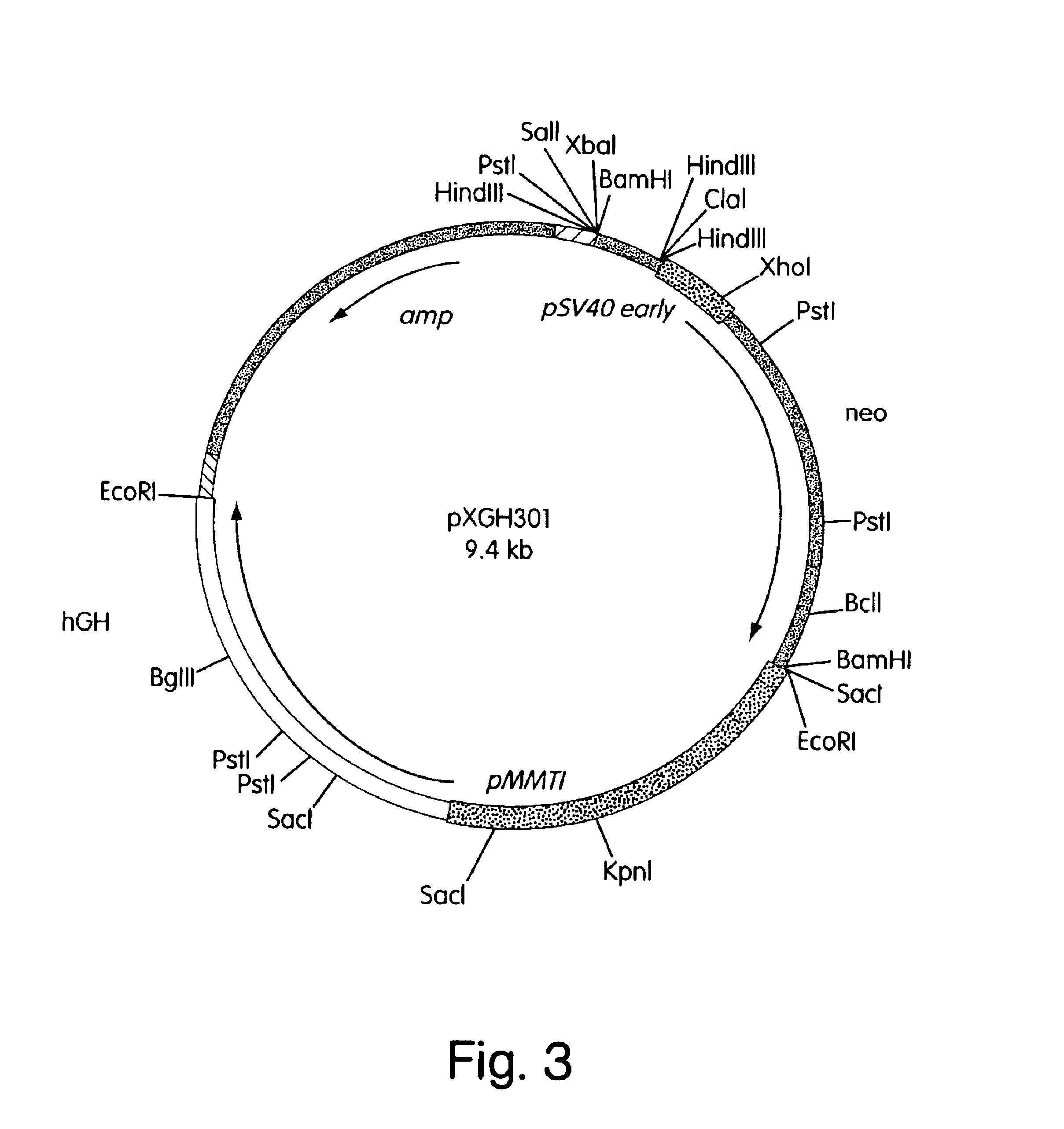
Glycosylation analogs of erythropoietin
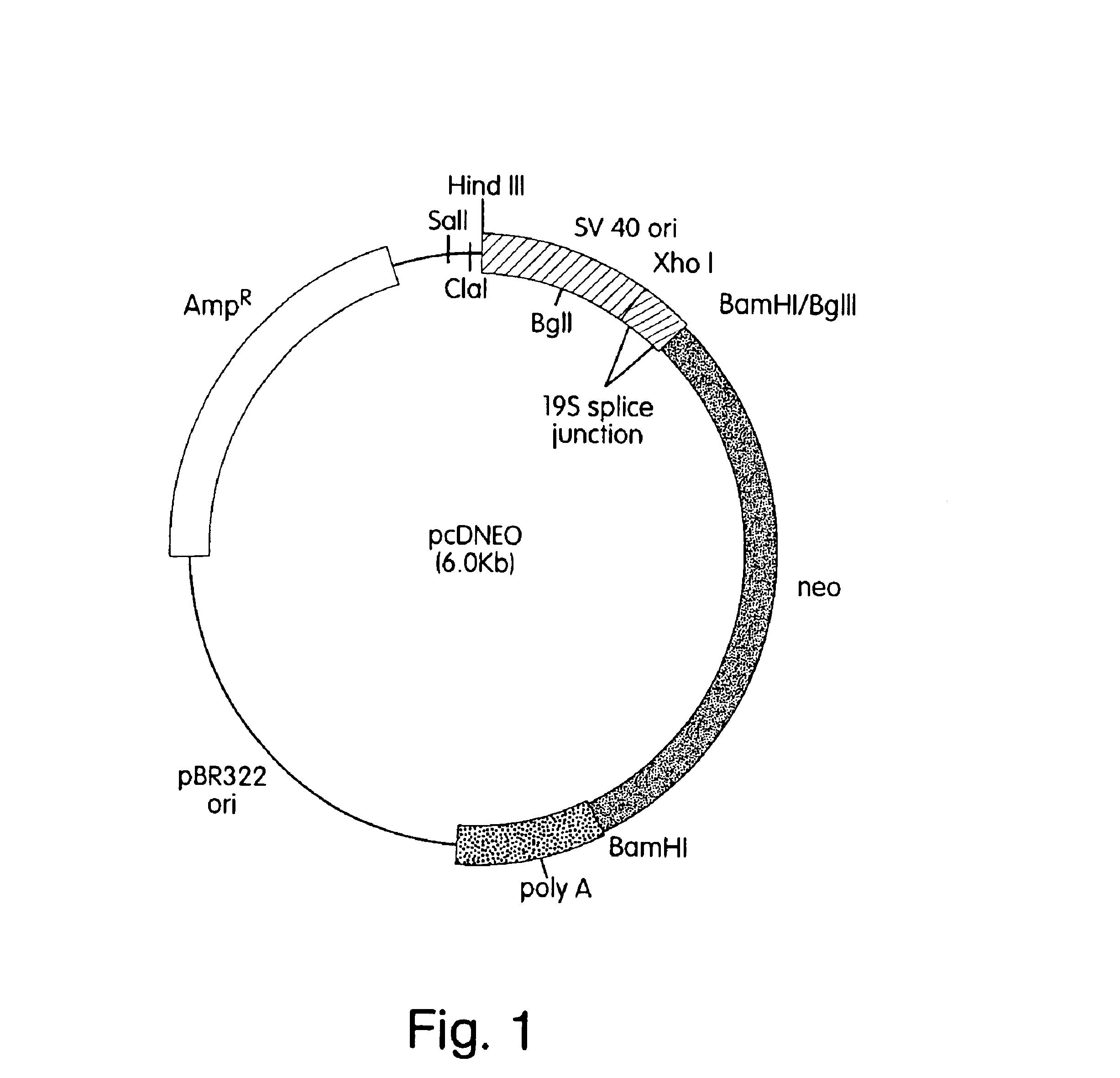
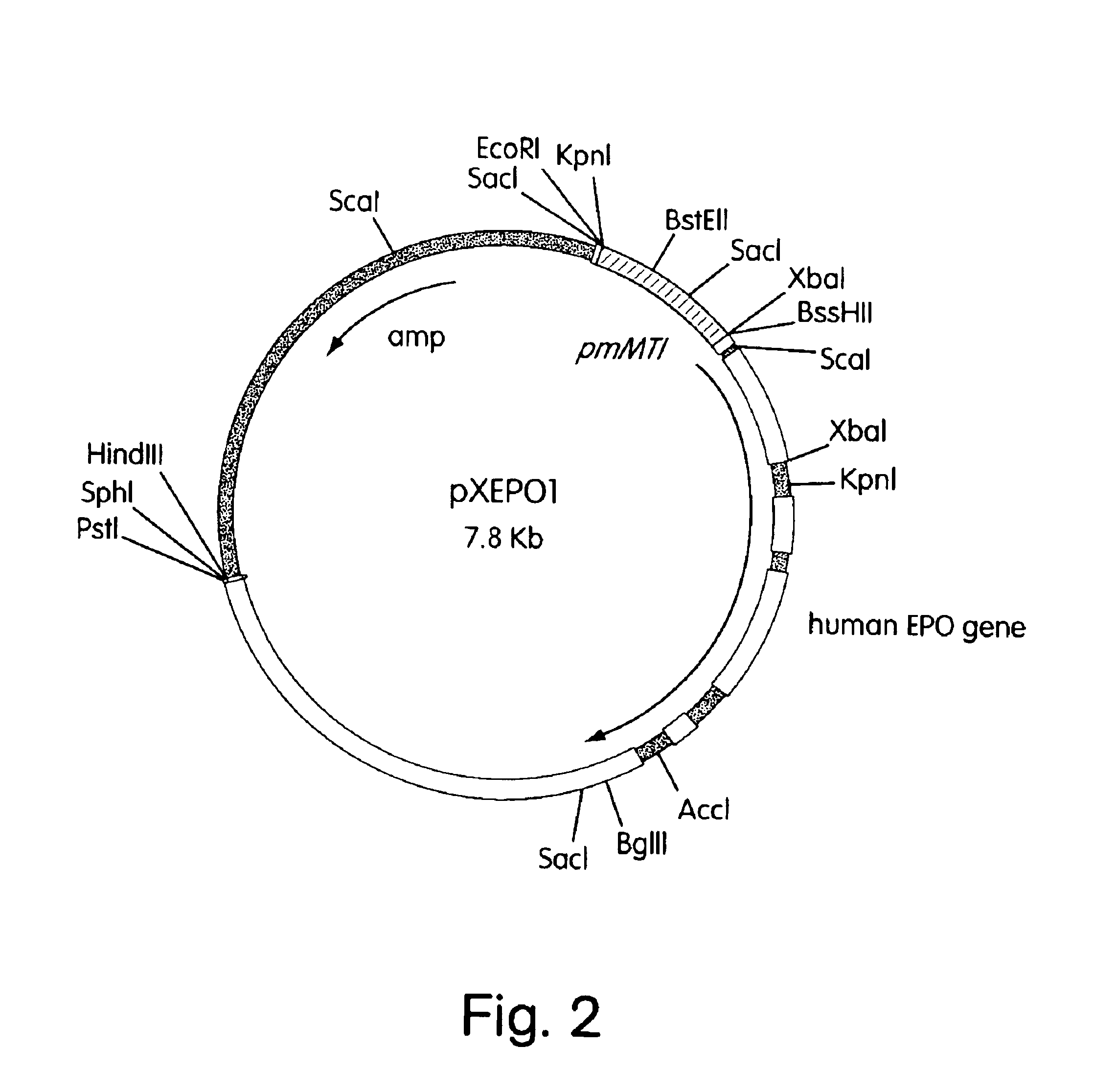
ActiveUS7217689B1Increase the number ofHigh sialic acid contentPeptide/protein ingredientsTissue culturePlasmidDNA
Erythropoietin analogs having at least one additional site for glycosylation, or a rearrangement of at least one site for glycosylation are disclosed. The invention also relates to DNA sequences encoding said erythropoietin analogs, and recombinant plasmids and host cells for analog expression.
Owner:AMGEN INC
Fc-erythropoietin fusion protein with improved pharmacokinetics
InactiveUS20050192211A1Improve pharmacokineticsSimplify erythropoietin therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsErythropoietinNucleic acid
The present invention provides Fc-erythropoietin (“Fc-EPO”) fusion proteins with improved pharmacokinetics. Nucleic acids, cells, and methods relating to the production and practice of the invention are also provided.
Owner:MERCK PATENT GMBH
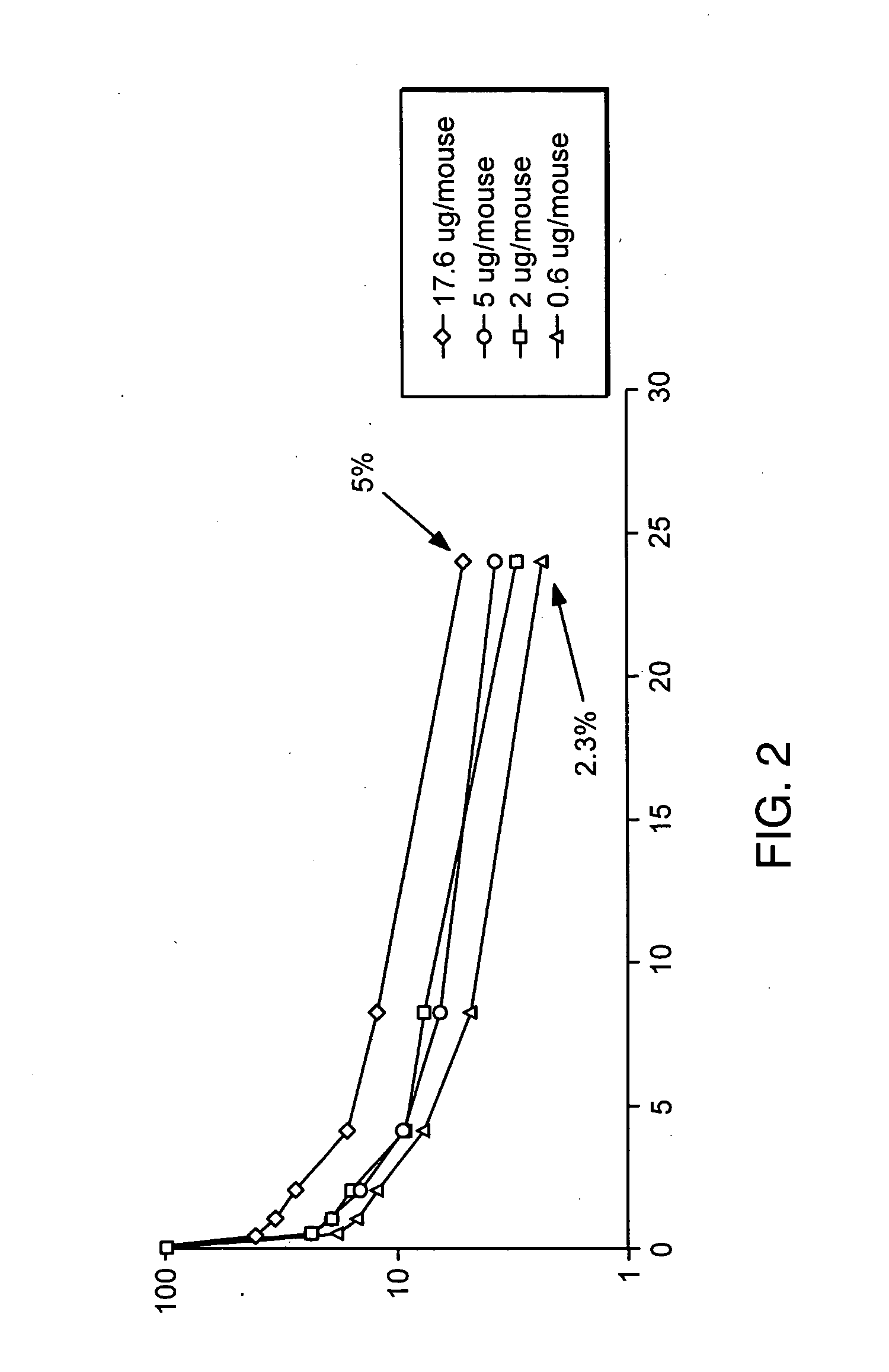
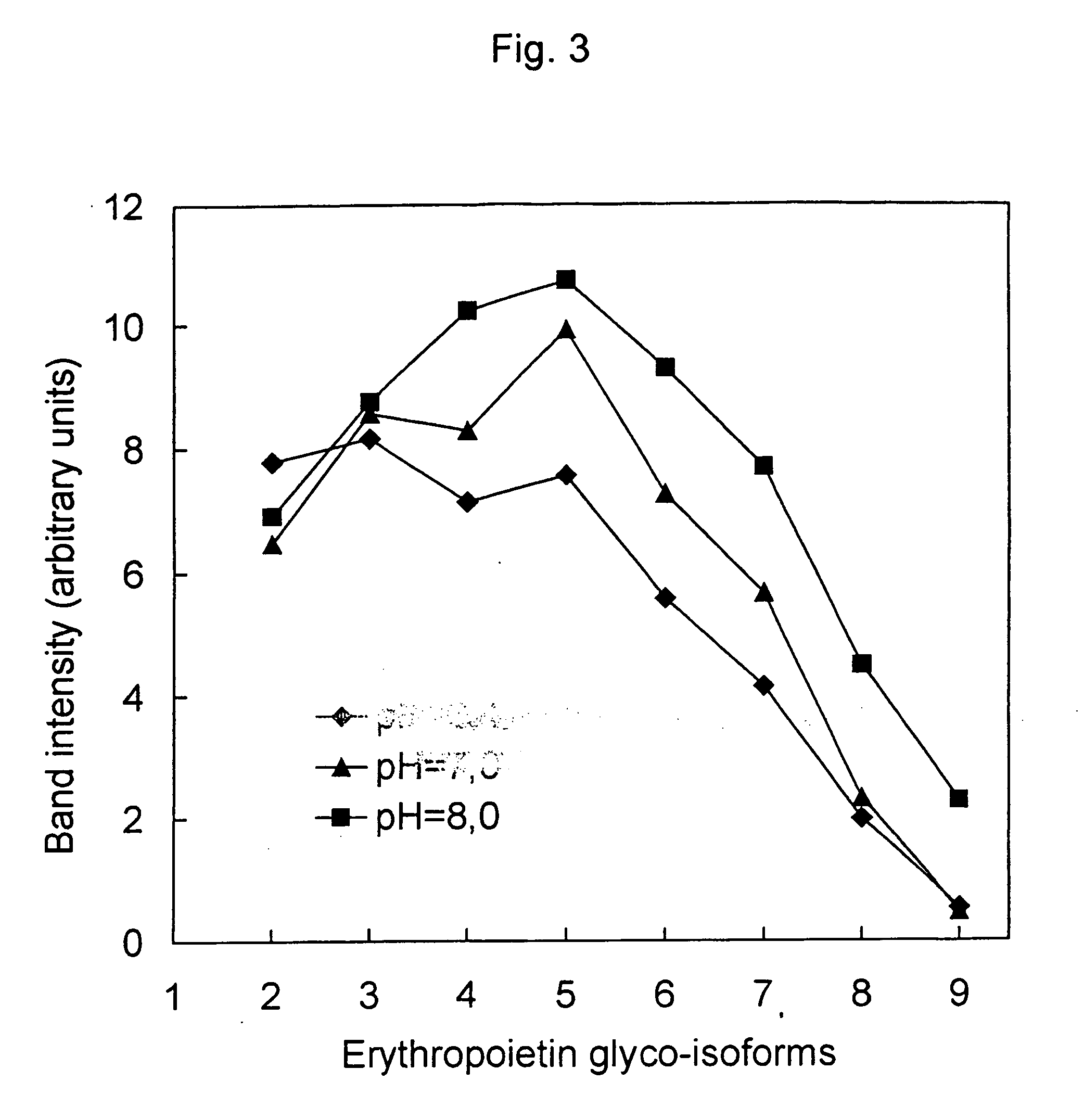
Process for the preparation of a desired erythropoietin glyco-isoform profile
InactiveUS20050153879A1High and uniform product specificityImprove product qualityPeptide/protein ingredientsComponent separationFiltrationRed blood cell
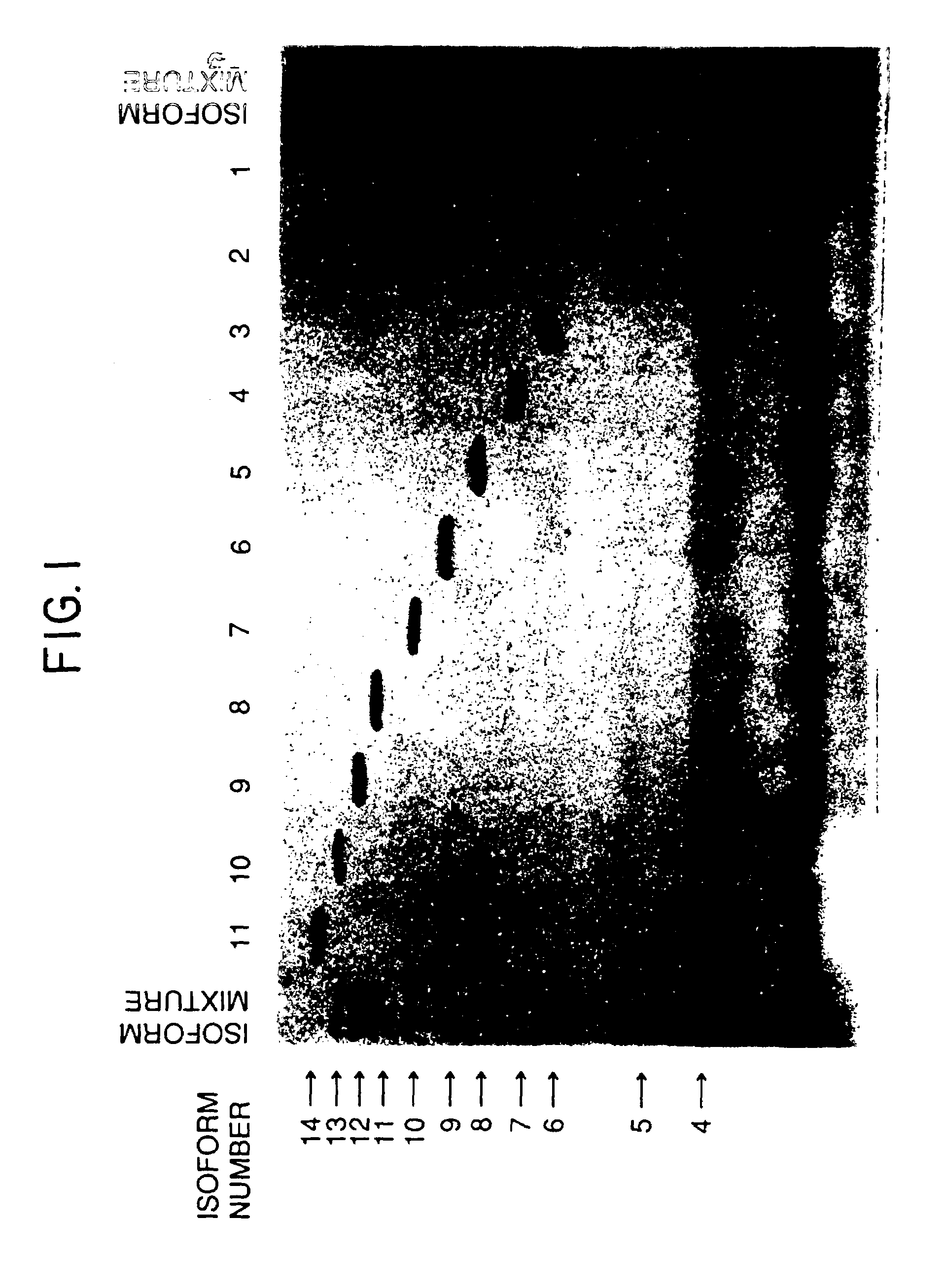
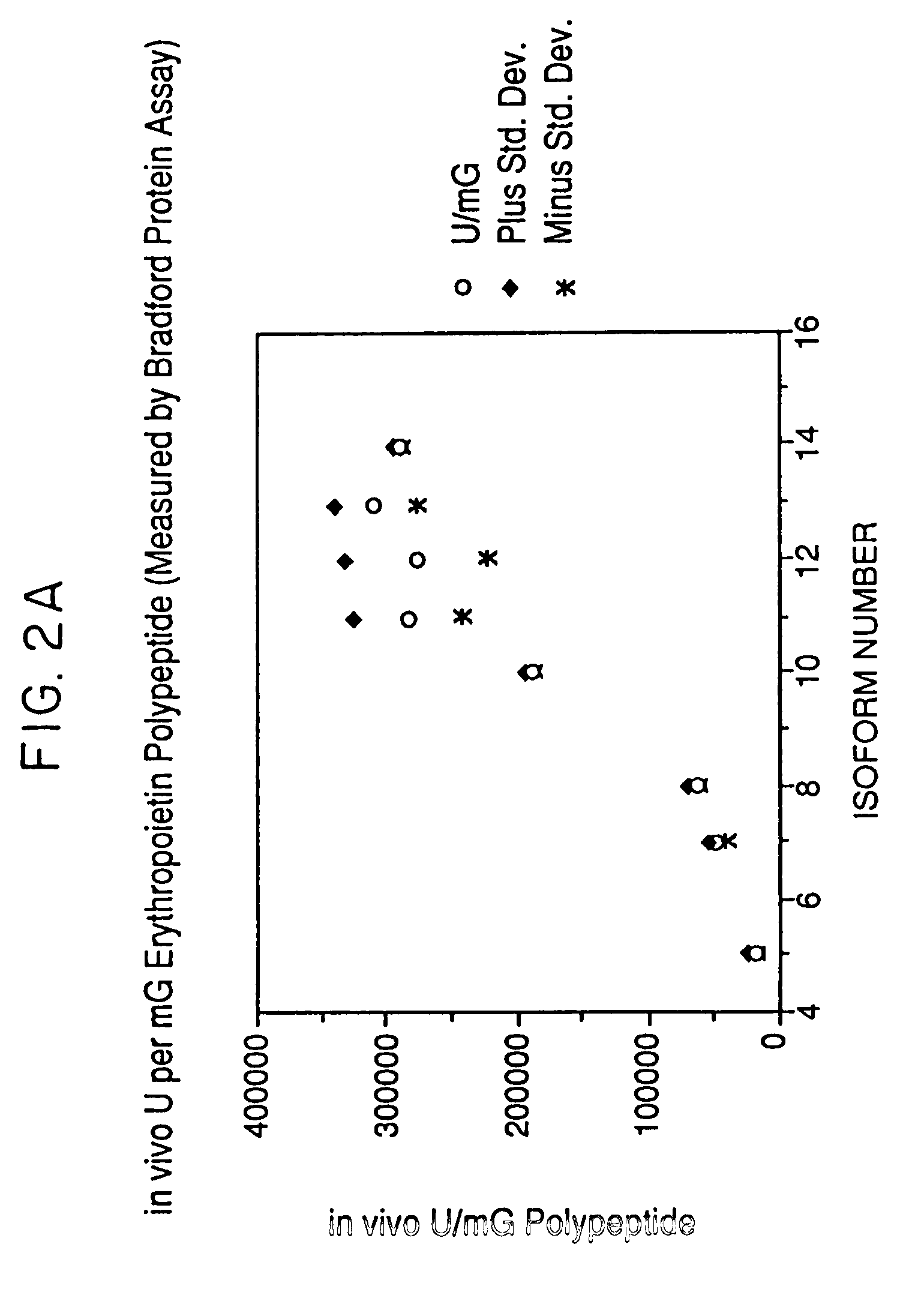
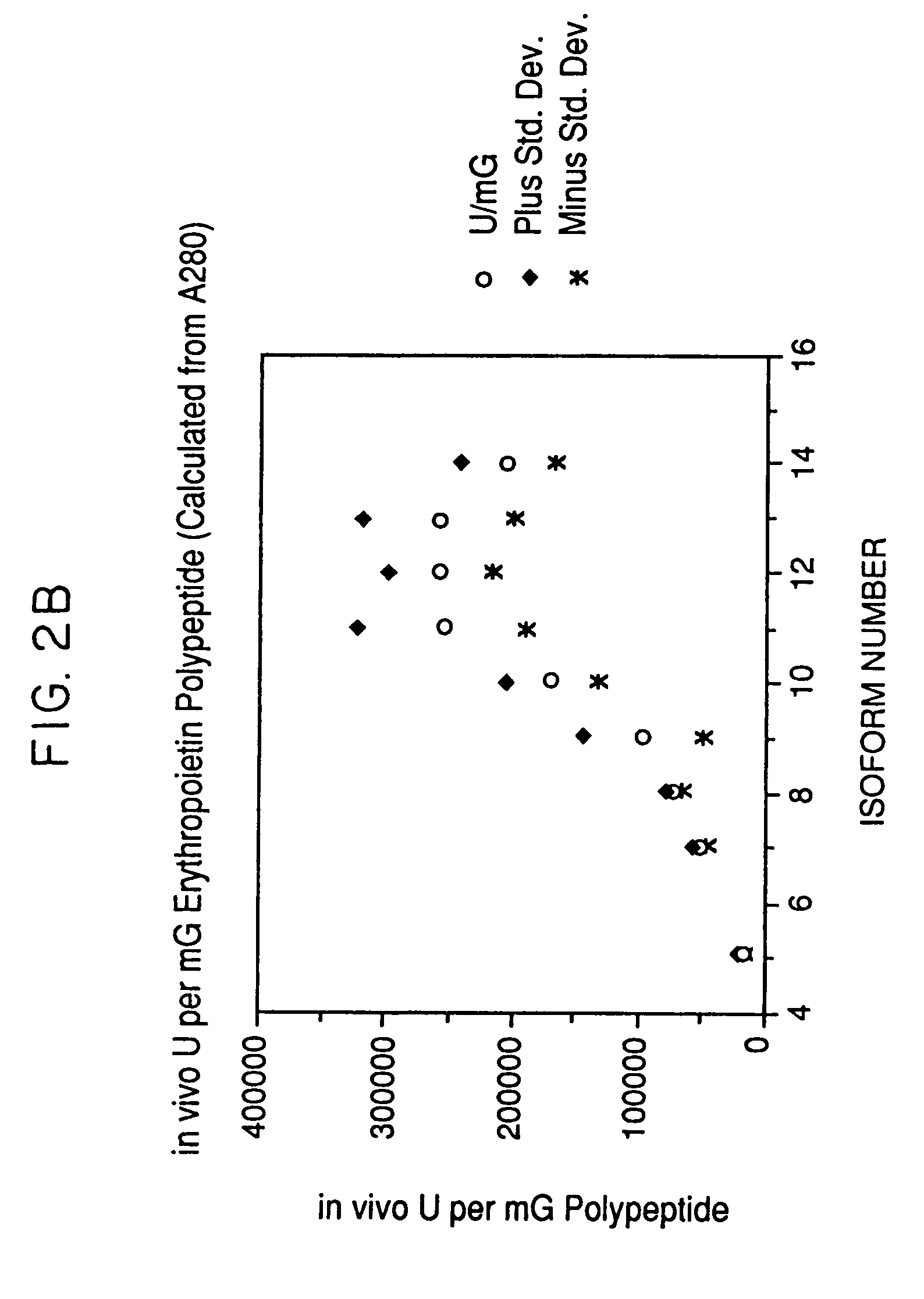
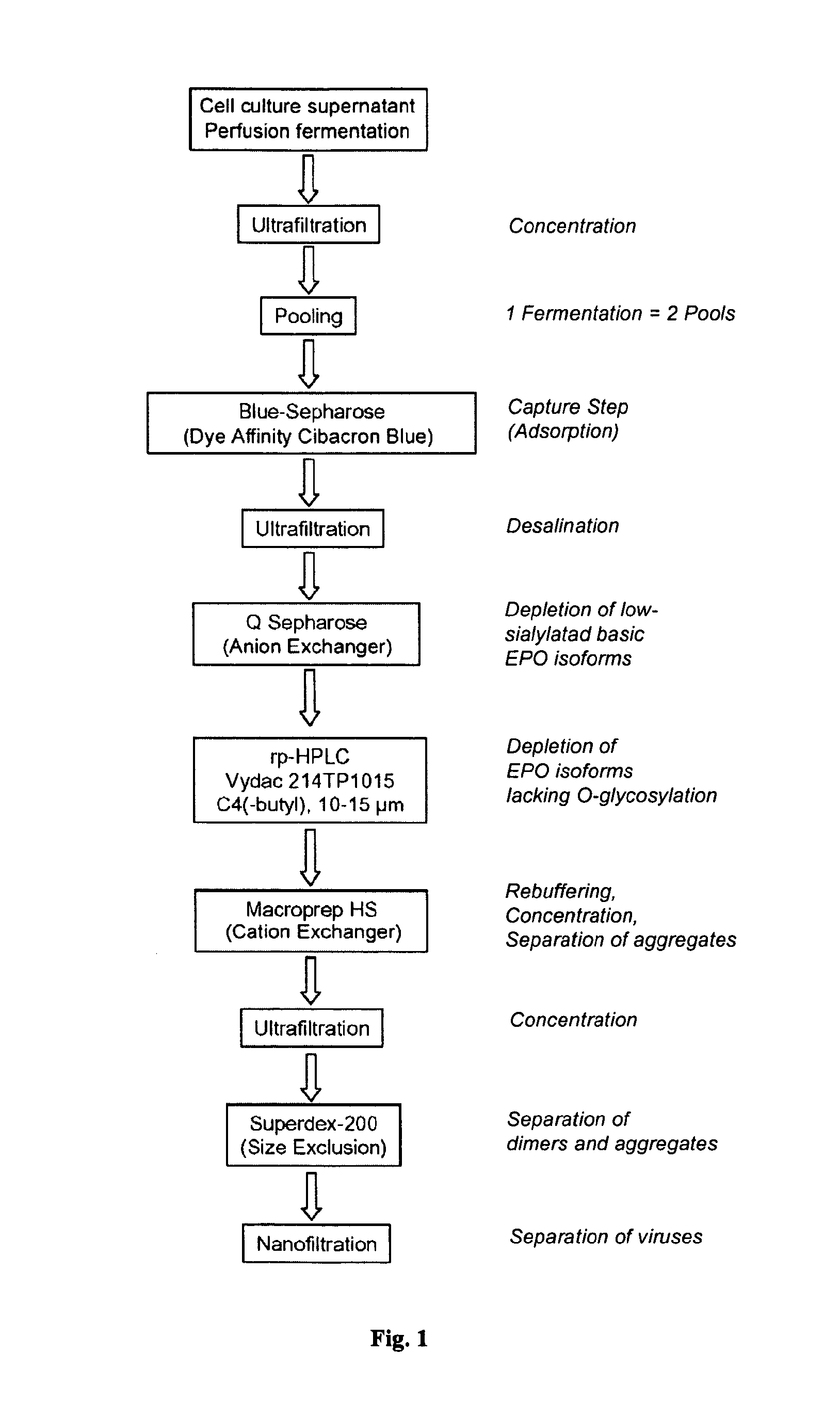
The present invention provides a process for the production of erythropoietin (EPO) with high purity and with a desired profile of EPO glycol-isoforms by using a combination of specific chromatographic steps in such a manner that the starting EPO glycol-isoform profile is changed or modified. The applied chromatographic steps includes at least (a) dye affinity chromatography, and (b) hydrophobic chromatography and / or (c) anion-exchange chromatography. In a preferred embodiment, the process further includes (d) gel filtration chromatography. The present invention also provides a process for the determination of erythropoietin (EPO) glycol-isoform profile in an EPO containing composition.
Owner:SVETINA MONICA +4
Tissue protective peptides and uses thereof
ActiveUS20090221482A1Avoid damageFacilitate optimal expressionSenses disorderPeptide/protein ingredientsDiseaseTissue protection
The present invention is directed to novel tissue protective peptides. The tissue protective peptides of the invention may bind to a tissue protective receptor complex. In particular, the present invention is drawn to tissue protective peptides derived from or sharing consensus sequences with portions of cytokine receptor ligands, including Erythropoietin (EPO), that are not involved in the binding of the ligand to the receptor complex, e.g., to the EPO receptor homodimer. Accordingly, the tissue protective peptides of the invention are derived from the amino acid sequences of regions of cytokine receptor ligands that are generally located on or within the region of the ligand protein that is opposite of the receptor complex, i.e., are generally derived from amino acid sequences of regions of the ligand protein that face away from the receptor complex while the ligand is bound to the receptor. The invention is further directed to the consensus sequences for use in engineering a synthetic tissue protective peptide. These tissue protective peptides also include fragments, chimeras, as well as peptides designed to mimic the spatial localization of key amino acid residues within the tissue protective receptor ligands, e.g., EPO. The invention further encompasses methods for treating or preventing a disease or disorder using tissue protective peptides of the current invention. The invention also encompasses methods for enhancing excitable tissue function using tissue protective peptides of the current invention.
Owner:ARAIM PHARMA INC
Modified animal erythropoietin polypeptides and their uses
InactiveUS20100093608A1Increasing therapeutic half-lifeIncreased serum half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsRed CellIndividual animal
Owner:ELANCO US INC +1
Triazolopyridine compound, and action thereof as prolyl hydroxylase inhibitor or erythropoietin production-inducing agent
ActiveUS20110077267A1Easy to produceReduce productionBiocideGroup 5/15 element organic compoundsDiseaseMedicine
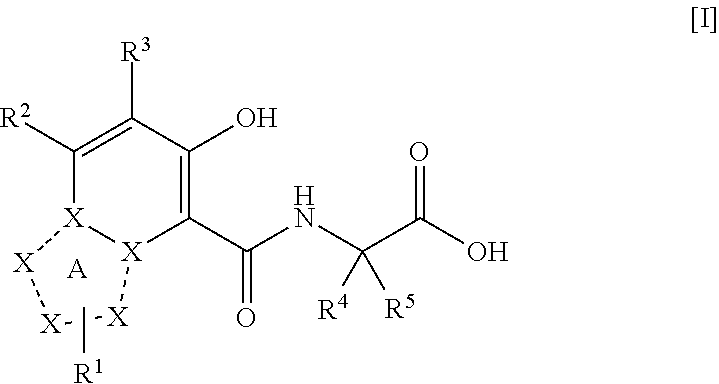
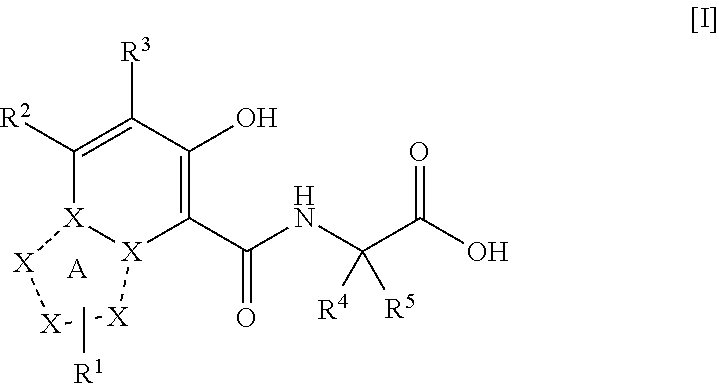
The present invention provides a triazolopyridine compound having a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability. The present invention relates to a compound represented by the following formula [I]:wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof, or a solvate thereof, as well as a prolyl hydroxylase inhibitor or erythropoietin production-inducing agent containing the compound. The compound of the present invention shows a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability and is useful as a prophylactic or therapeutic agent for various diseases and pathologies (disorders) caused by decreased production of erythropoietin.
Owner:JAPAN TOBACCO INC
Fusion protein having enhanced in vivo erythropoietin activity
ActiveUS7091326B2Enhanced human EPO activityProlong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsRed blood cellHalf-life
Provided is a fusion protein comprising, at its carboxy terminal of human erythropoietin (EPO), a mutant having one to four amino acid substitutions in the carboxy terminal peptide (CTP) fragment of a human chorionic gonadotropin (HCG) β subunit, for increasing an in vivo half-life activity of EPO. The in vivo half-life can be greatly elongated while retaining the intrinsic activity of the EPO, without increasing the sugar chain content.
Owner:CJ HEALTHCARE CORP
T-cell epitopes in erythropoietin
InactiveUS20060035322A1Reduced activityReduce eliminatePeptide/protein ingredientsTissue cultureVaccine ImmunogenicityPeptide
The invention relates to the identification of epitopes for T-cells in human EPO as well as T-cell epitope peptides derived from EPO by means of which it is possible to create novel modified EPO variants with reduced immunogenicity.
Owner:MERCK PATENT GMBH
Modified animal erythropoietin polypeptides and their uses
InactiveUS8278418B2High affinityImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsRed CellErythropoietin
Owner:ELANCO US INC +1
Tissue protective cytokine receptor complex and assays for identifying tissue protective compounds
InactiveUS20040214236A1High activityEnhanced interactionSenses disorderNervous disorderNervous systemExcitable cell
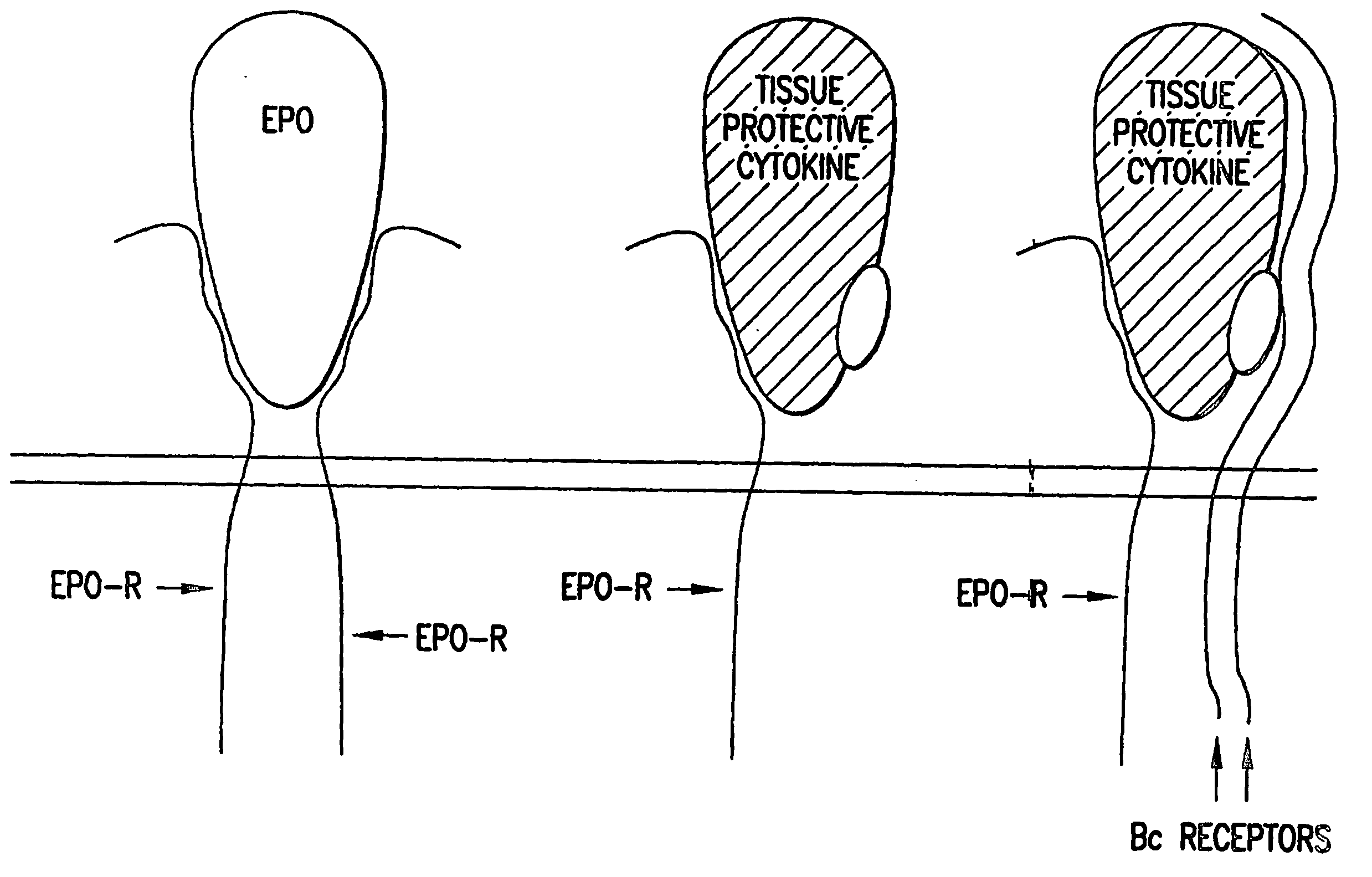
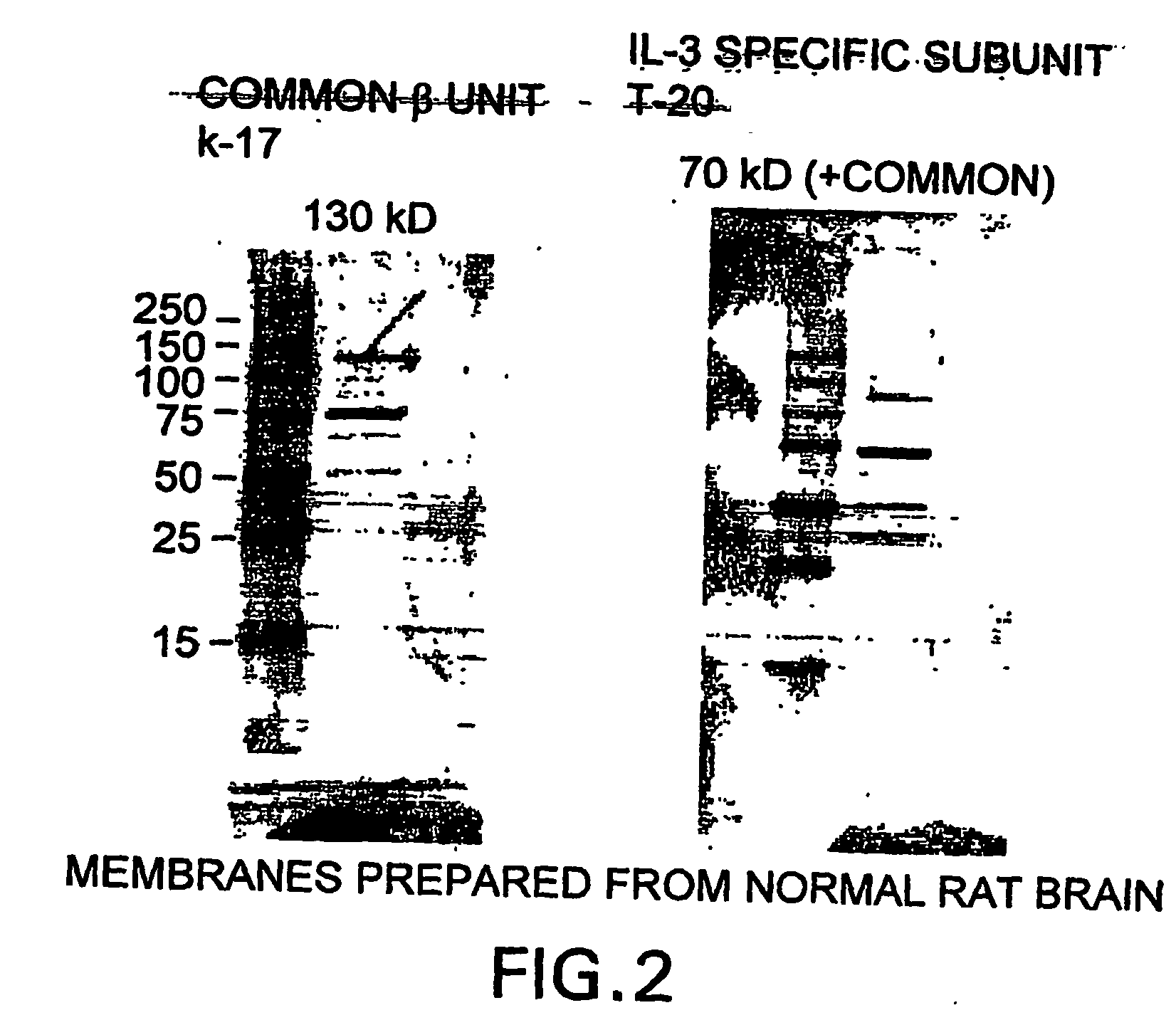
The present invention is directed methods for identifying compounds that have a tissue protective activity using a heteromultimer receptor complex that mediates the tissue protective activities. The complex consists of at least one EPO-R in complex with at least one betac Receptor. These compounds used in the assays to identify tissue protective compounds include, but are not limited to, small molecules and biologics. The compounds identified using these assays can be used to treat various conditions of the central and peripheral nervous systems as well as those of other erythropoietin-responsive or excitable cells, tissues, and organs.
Owner:H LUNDBECK AS +2
Tissue protective cytokine receptor complex, assays for identifying tissue protective compounds and uses thereof
InactiveUS20060216757A1Reduce apoptosisPromote recoveryCompound screeningPeptide librariesNervous systemExcitable cell
The present invention is directed methods for identifying compounds that have a tissue protective activity using a heteromultimer receptor complex that mediates the tissue protective activities. The complex consists of at least one EPO-R in complex with at least one βc Receptor. These compounds used in the assays to identify tissue protective compounds include, but are not limited to, small molecules and biologics. The compounds identified using these assays can be used to treat or prevent various diseases, disorders, or conditions of the central and peripheral nervous systems as well as those of other erythropoietin-responsive or excitable cells, tissues, and organs.
Owner:THE KENNETH S WARREN INST
Medicinal uses of hydrazones
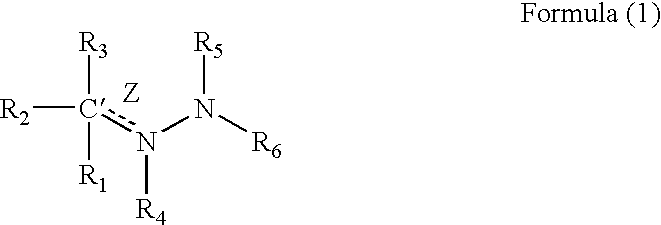
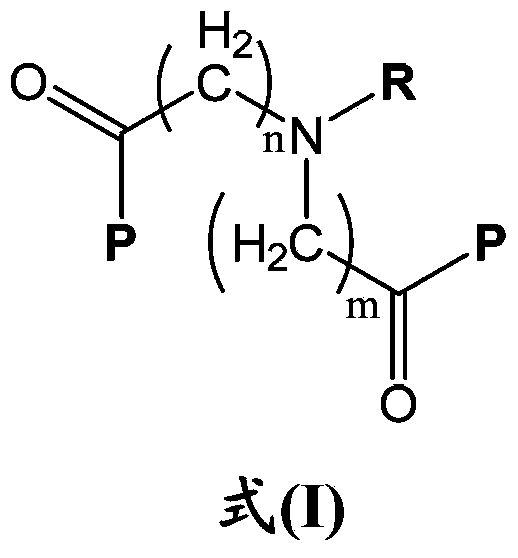
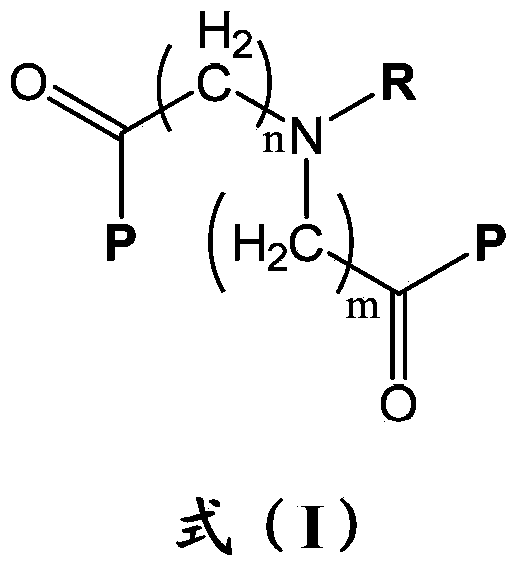
InactiveUS20030092716A1Increase transcriptionImprove biological activityBiocideNervous disorderGynecologyHydrazone
Compounds having a structure according to Formula (I): are effective in a method of increasing erythropoietin and vascularization of tissue in a subject in need thereof.
Owner:THE PROCTER & GAMBLE COMPANY
Modified erythropoietin polypeptides and uses thereof for treatment
ActiveCN101678079AIncrease protease resistanceOrganic active ingredientsHeavy metal active ingredientsErythroid cellTreatment use
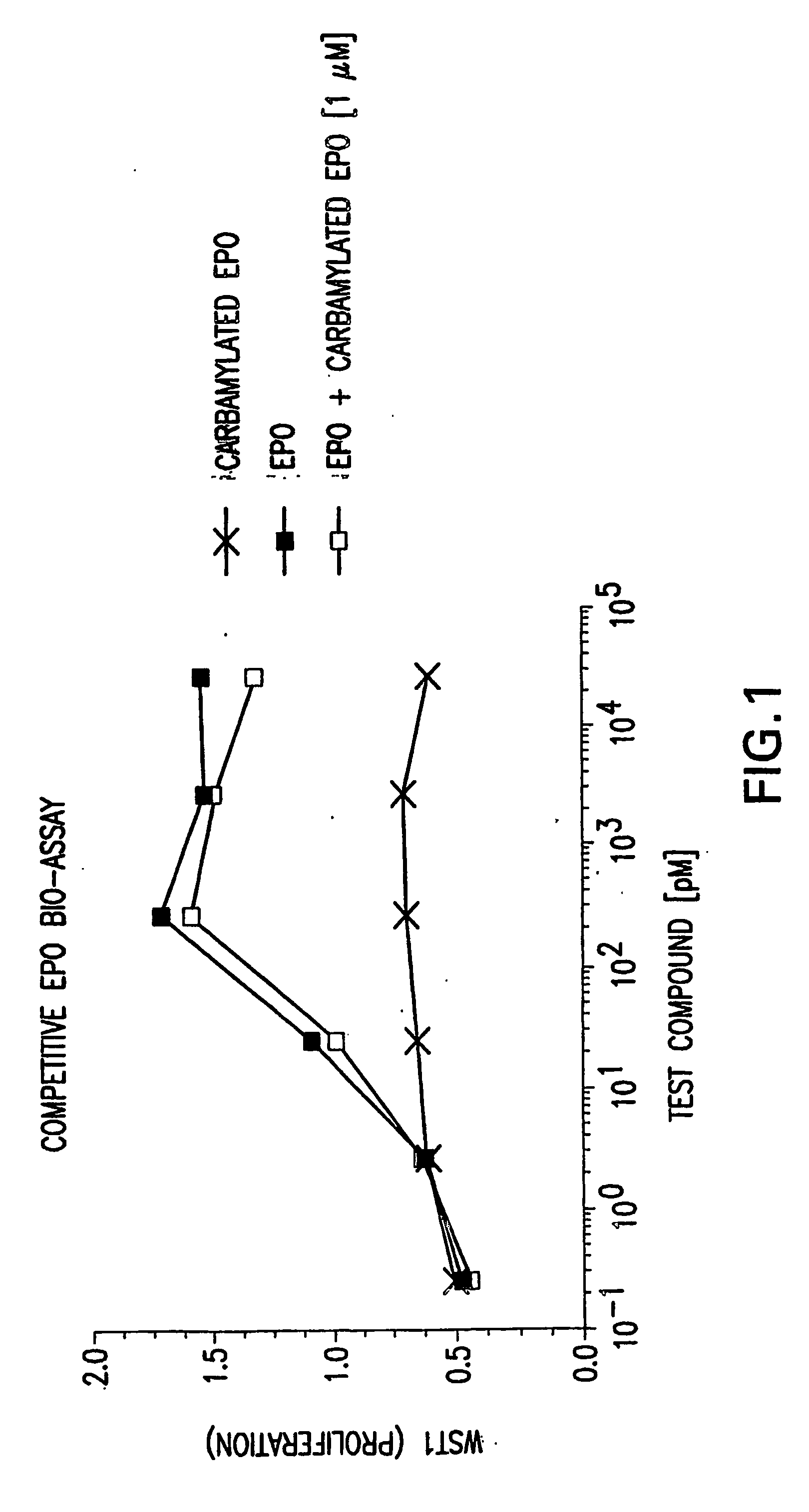
Modified erythropoietin (EPO) polypeptides and other modified therapeutic polypeptides are provided. The EPO polylpeptides and other modified therapeutic polypeptides are modified to exhibit physical properties and activities that differ from the corresponding unmodified EPO polypeptides and other unmodified therapeutic polypeptides, respectively. Nucleic acid molecules encoding these polypeptides also are provided. Also provided are methods of treatment and diagnosis using the polypeptides.
Owner:韩诺生物制约株式会社
Erythropoietin derivatives with altered immunogenicity
InactiveUS20060073563A1Reduced binding affinityMaintain activityPeptide/protein ingredientsGenetic material ingredientsVaccine ImmunogenicityErythropoietin
Owner:XENCOR
Organoids comprising isolated renal cells and use thereof
InactiveUS20160101133A1BiocidePharmaceutical delivery mechanismBlood Vessel EndotheliumErythroid cell
Described herein are organoids comprising admixtures of selected bioactive primary renal cells and a bioactive cell population, e.g., an endothelial cell populations, e.g. HUVEC cells, and methods of treating a subject in need thereof with such organoids. Further, the isolated renal cells, which may include tubular and erythropoietin {EPO}-producing kidney cell populations, and / or the endothelial cell populations may be of autologous, syngeneic, allogeneic or xenogeneic origin, or any combination thereof. Further provided are methods of treating a subject in need with the organoids.
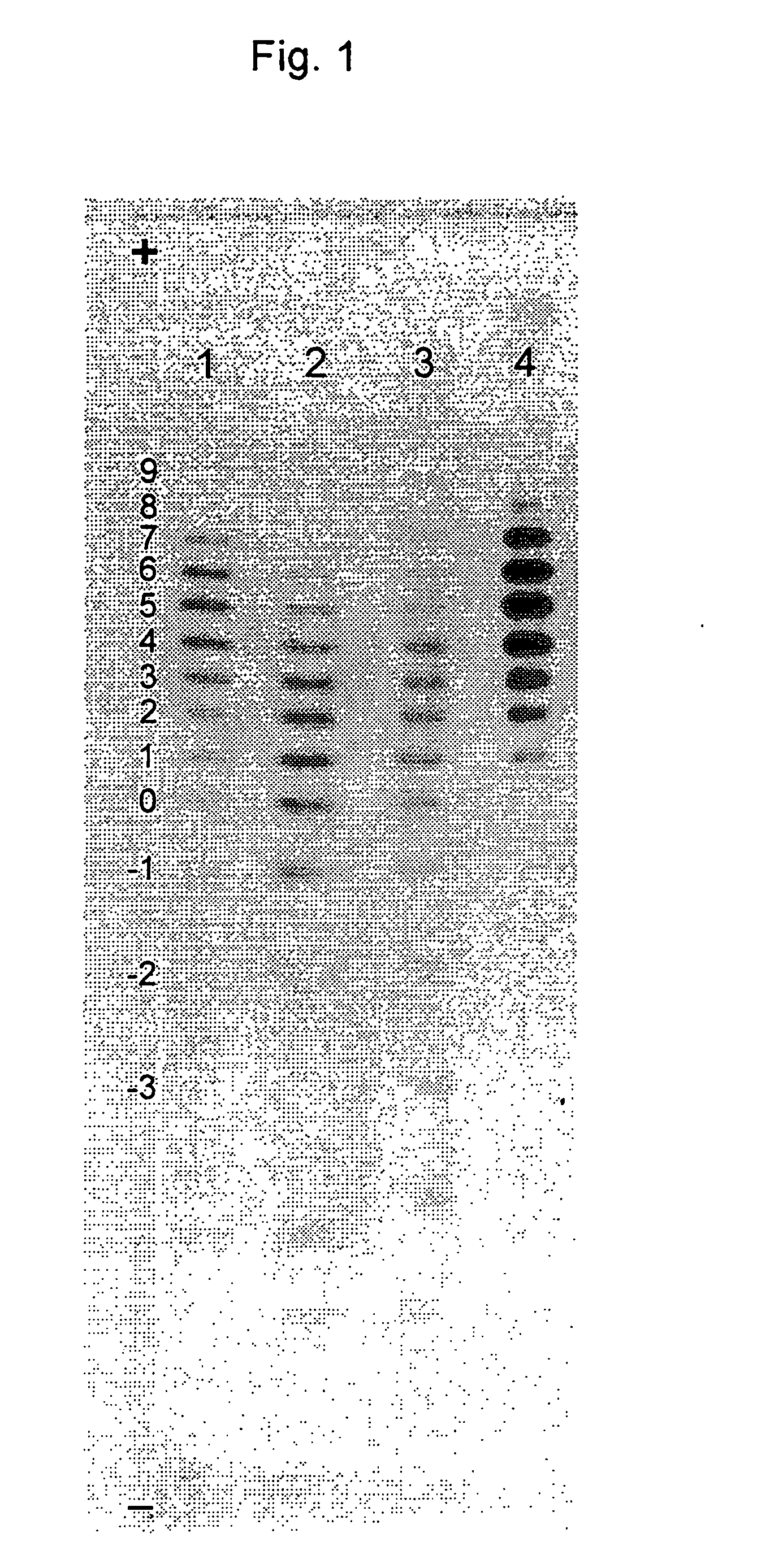
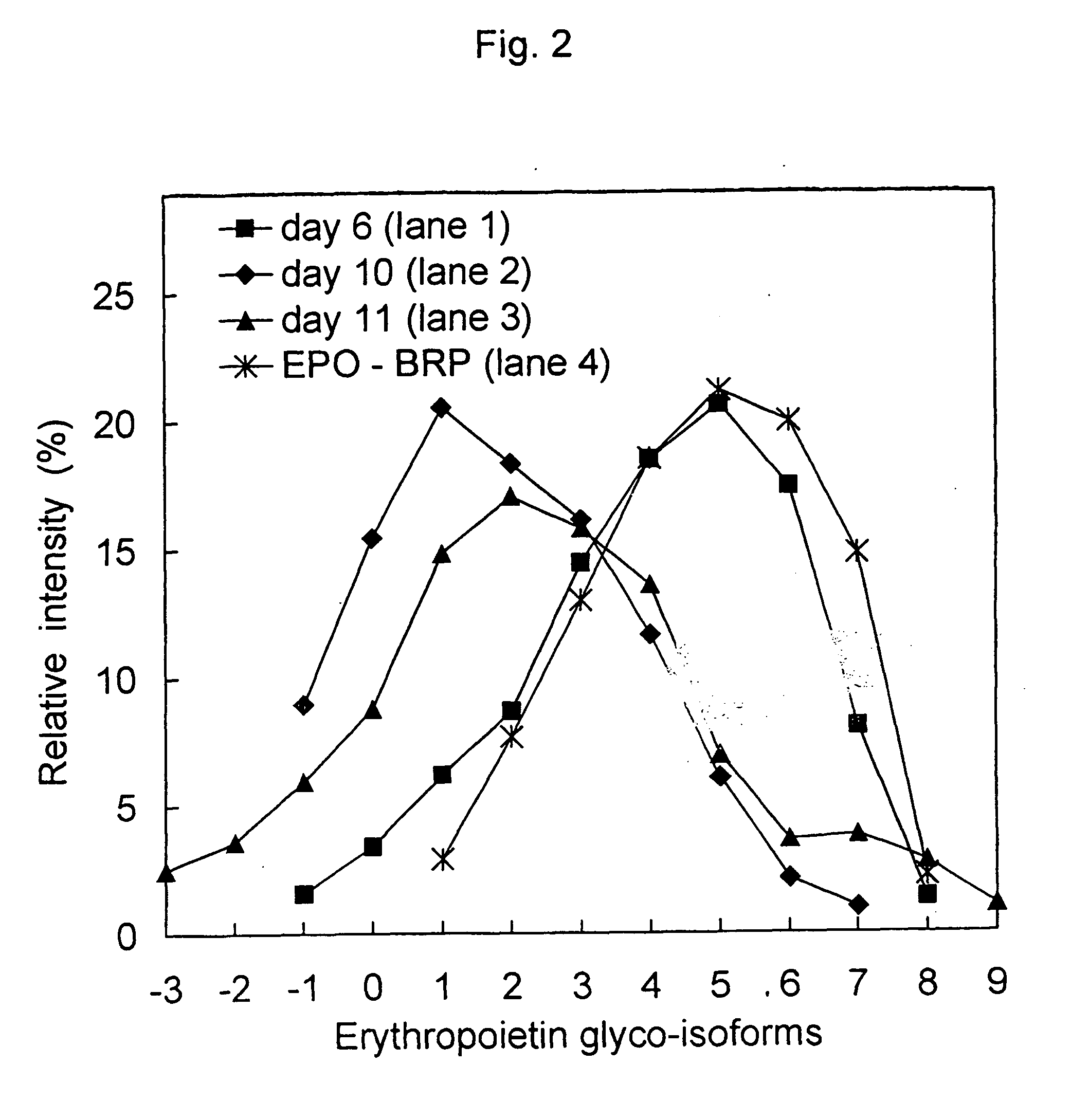
Process for the purification of recombinant human erythropoietin (EPO), epo thus purified and pharmaceutical compositions comprising same
InactiveUS20120264688A1Increase virus safetyEffective stepPeptide/protein ingredientsPeptide preparation methodsMedicineRed blood cell
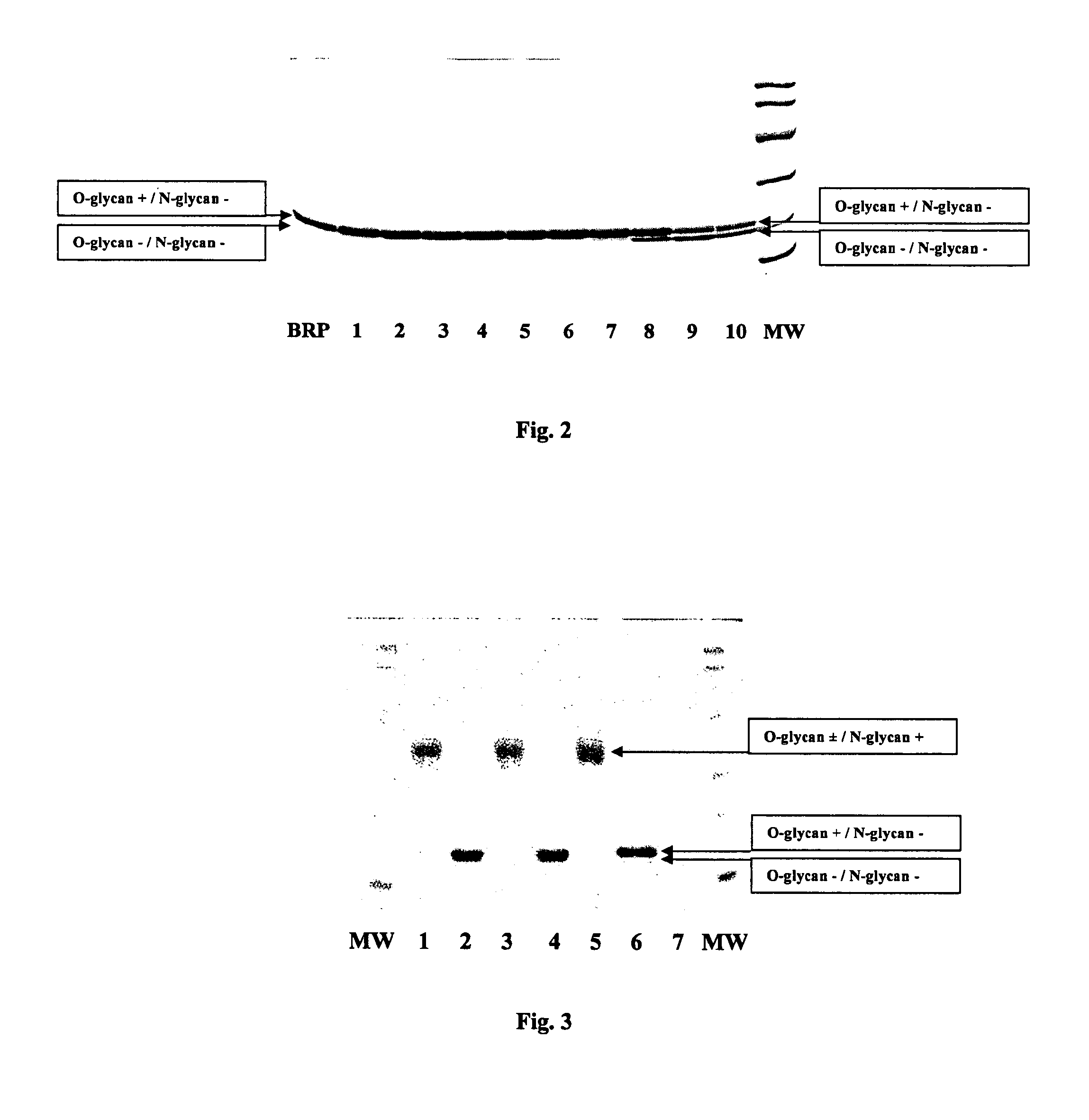
A procedure for the production of erythropoietin (EPO), in particular recombinant human EPO (rhEPO) with a defined composition of glycoforms in a highly pure form, i.e., with a high amount of O-glycosylated EPO isoforms is provided.
Owner:RATIOPHARM GMBH
Tissue Protective Cytokine Receptor Complex, Assays for Identifying Tissue Protective Compounds and Uses Thereof
InactiveUS20090136519A1Easy to measureFunction increasePeptide/protein ingredientsMicrobiological testing/measurementNervous systemExcitable cell
Owner:H LUNDBECK AS +1
Erythropoietin mimetic peptide chemical dimer and use thereof
The invention relates to an erythropoietin mimetic peptide chemical dimer and a use thereof. In particular, the invention relates to the erythropoietin mimetic peptide chemical dimer represented by the formula (I) or a pharmaceutically acceptable salt thereof, and a composition comprising the erythropoietin mimetic peptide chemical dimer or the pharmaceutically acceptable salt thereof. The invention further relates to the use of the erythropoietin mimetic peptide chemical dimer or the pharmaceutically acceptable salt thereof. The erythropoietin mimetic peptide chemical dimer or the pharmaceutically acceptable salt thereof has a better erythropoietic generating activity.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
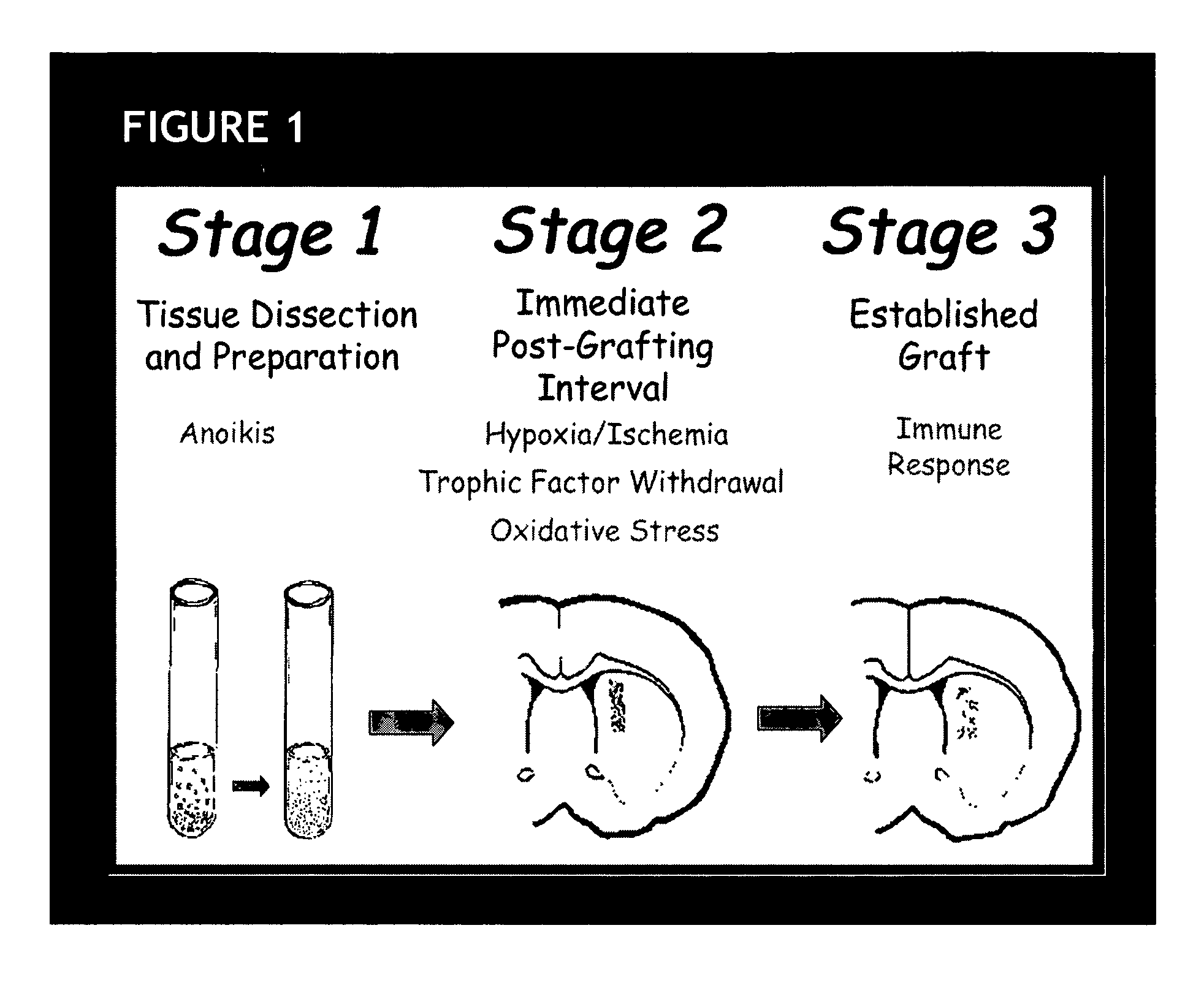
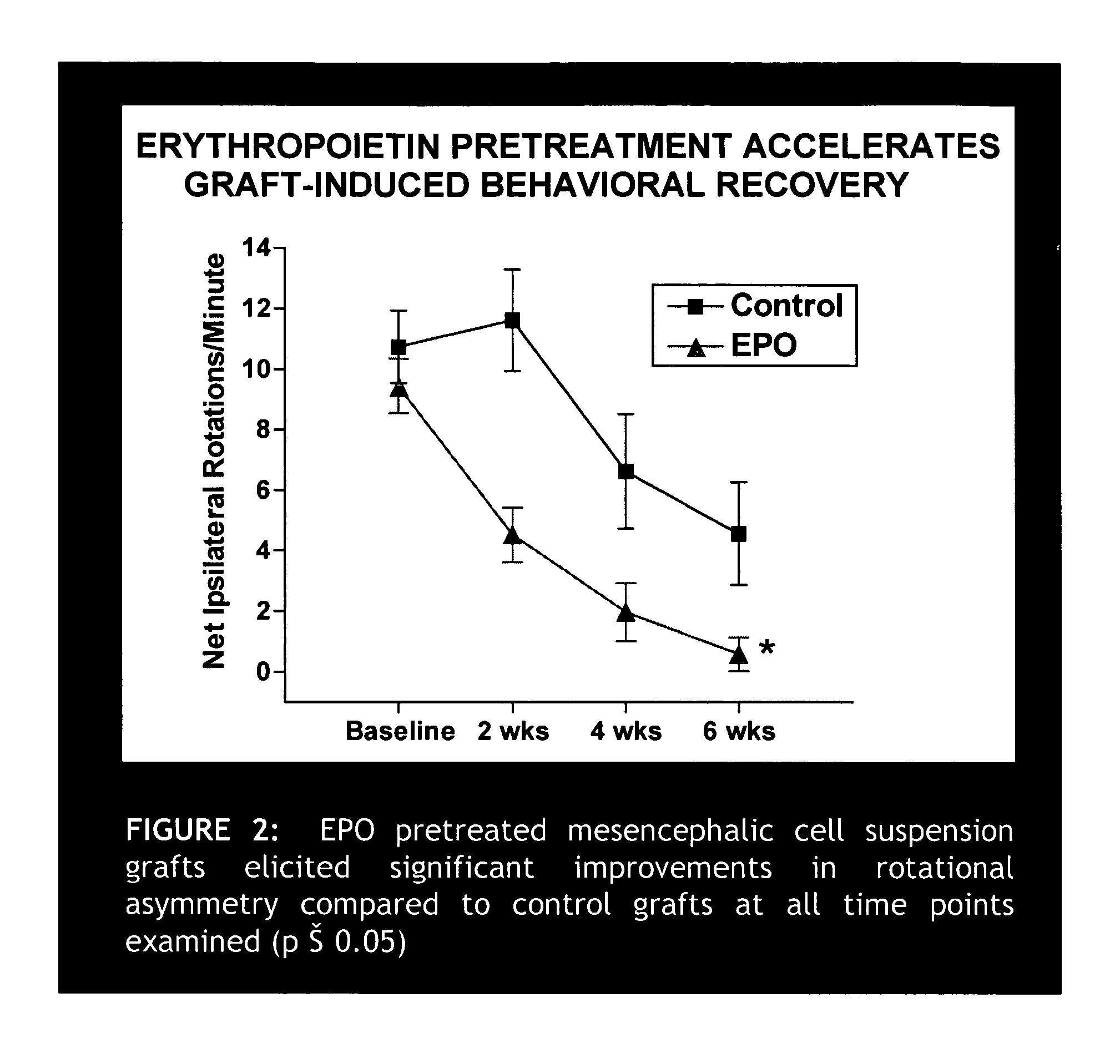
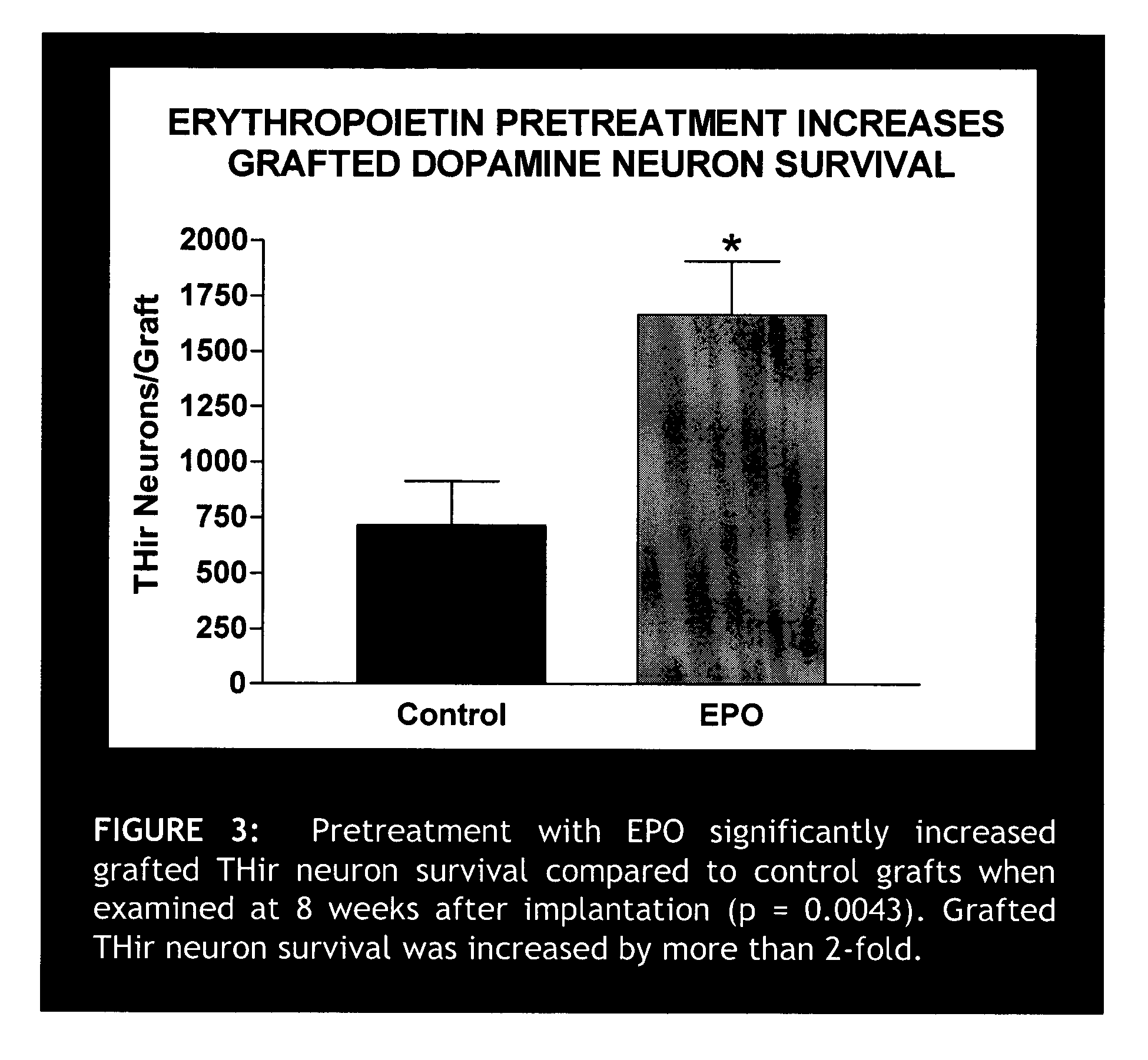
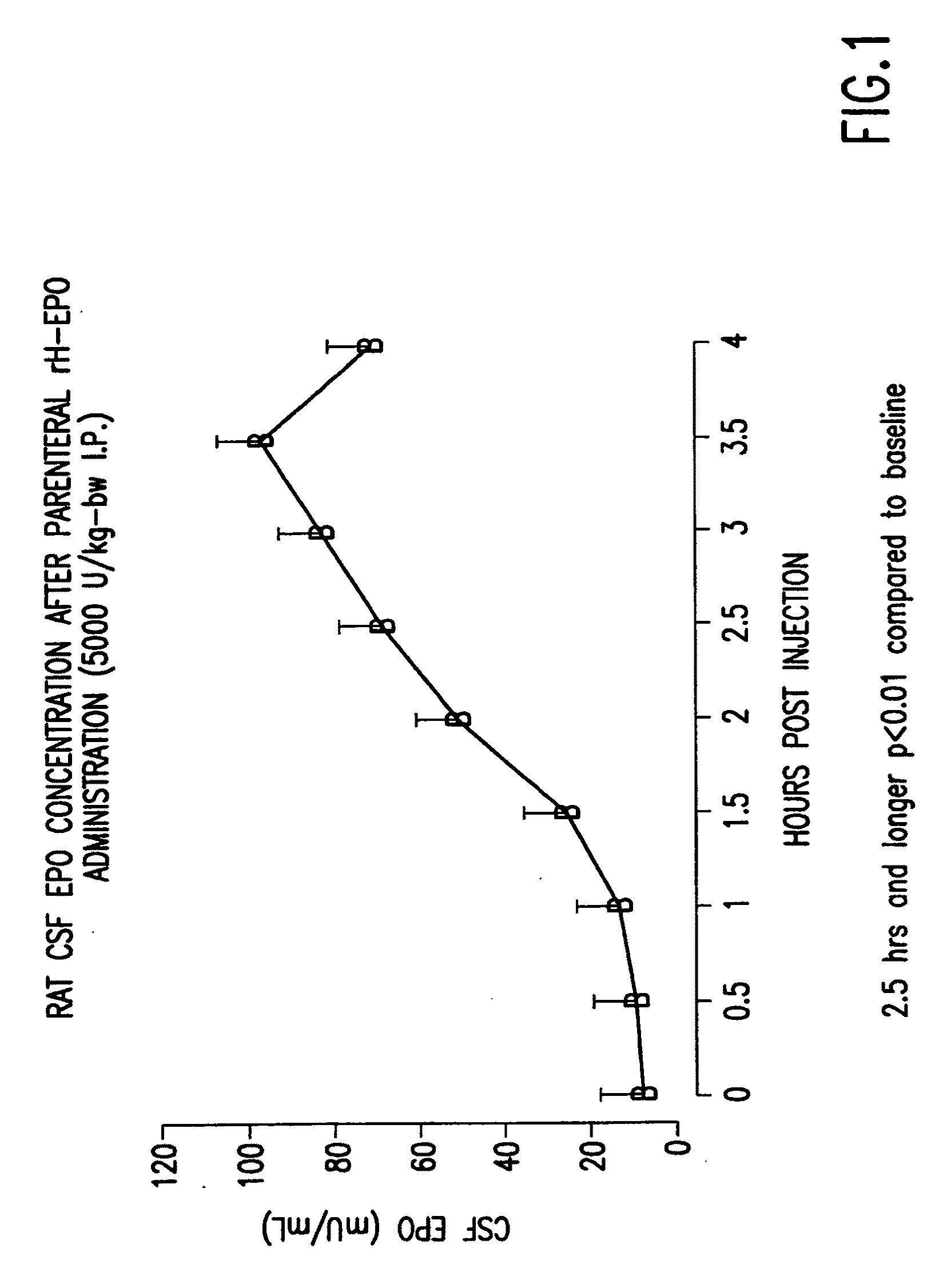
Erythropoietin administration to improve graft survival
ActiveUS7459152B2Enhance cell viabilityImprove survivalBiocideNervous disorderErythropoietinGraft survival
The present invention provides methods, compounds and kits for increasing the viability of cells. The methods involve treating cells that make up a tissue graft with erythropoietin before, during or after delivery or administration. The method can employ cells of different types, including cells of neural or paraneural origin, such as adrenal chromaffin cells. Also useful are cell lines grown in vitro. Cells not of neural or paraneural origin, such as fibroblasts, may also be used following genetic alteration to express a desired neural product such as a neurotransmitter or a neuronal growth factor. The method is used to treat neurological diseases such as Parkinson's disease, Alzheimer's disease, Huntington's disease, epilepsy, and traumatic brain or spinal cord injury.
Owner:RUSH UNIV MEDICAL CENT
Protection, restoration, and enhancement of erythropoietin-responsive cells, tissues and organs
InactiveUS20090233844A1Restoration and of dysfunctionImprove survivalHormone peptidesSenses disorderErythropoietin receptor activityCell organisation
Methods and compositions are provided for protecting or enhancing an erythropoietin-responsive cell, tissue, organ or body part function or viability in vivo, in situ or ex vivo in mammals, including human beings, by systemic or local administration of an erythropoietin receptor activity modulator, such as an erythropoietin or a modified erythropoietin.
Owner:THE KENNETH S WARREN INST
Modified erythropoietin (epo)polypeptides that exhibit increased protease resistance and pharmaceutical compositions thereof
Modified erythropoietin (EPO) polypeptides and other modified therapeutic polypeptides are provided. The EPO polypeptides and other therapeutic polypeptides are modified to exhibit physical properties and activities that differ from the unmodified EPO polypeptides and other unmodified therapeutic polypeptides, respectively. Nucleic acid molecules encoding these polypeptides also are provided. Also provided are methods of treatment and diagnosis using the polypeptides.
Owner:韩诺生物制约株式会社
Erythropoietin receptor modified electrode, preparation method and applications thereof
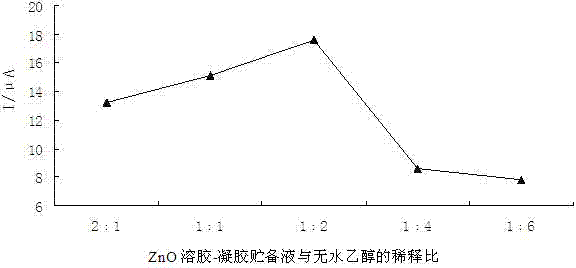
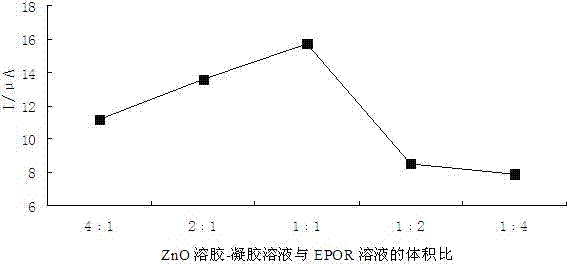
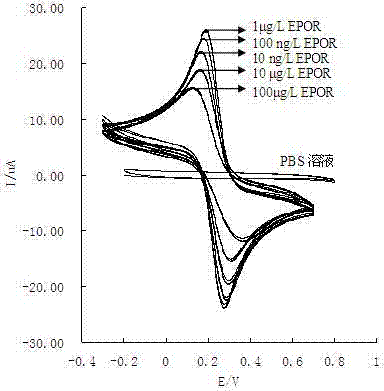
ActiveCN102854231AEasy to prepareImprove performancePretreated surfacesCoatingsElectrochemical biosensorErythroid cell
The present invention discloses an erythropoietin receptor modified electrode, wherein an electrode having an erythropoietin receptor fixed on the surface through ZnO sol-gel is adopted as a glassy carbon electrode for element recognition, the modified electrode has characteristics of simple preparation method and stable performance, and a response current value of the modified electrode remains about 77% of the initial value after placing in a dark place for 50 days at a temperature of 4 DEG C. According to the present invention, the modified electrode is adopted as a working electrode, a platinum electrode is adopted as a counter electrode, a saturated calomel electrode is adopted as a reference electrode, and a phosphate buffer solution containing 2 mmol / L of K3Fe(CN)6]-K4[Fe(CN)6] is adopted as a test base solution to establish an electrochemical biosensor so as to rapidly, specifically and sensitively detect erythropoietin (EPO) and / or recombinant human erythropoietin (rhEPO), wherein a linear range is 5 pg / L-500 ng / L, a detection limit is as low as 0.5 pg / L, particularly EPO and rhEPO can be accurately screened according to the peak potential, and the electrochemical biosensor is applicable for low concentration EPO or rhEPO detection, and stimulant rhEPO detection in sport competition.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Signal biomarkers
InactiveUS9103840B2Improve rendering capabilitiesEasy to detectPeptide/protein ingredientsImmunoglobulins against growth factorsErythroid cellBioinformatics
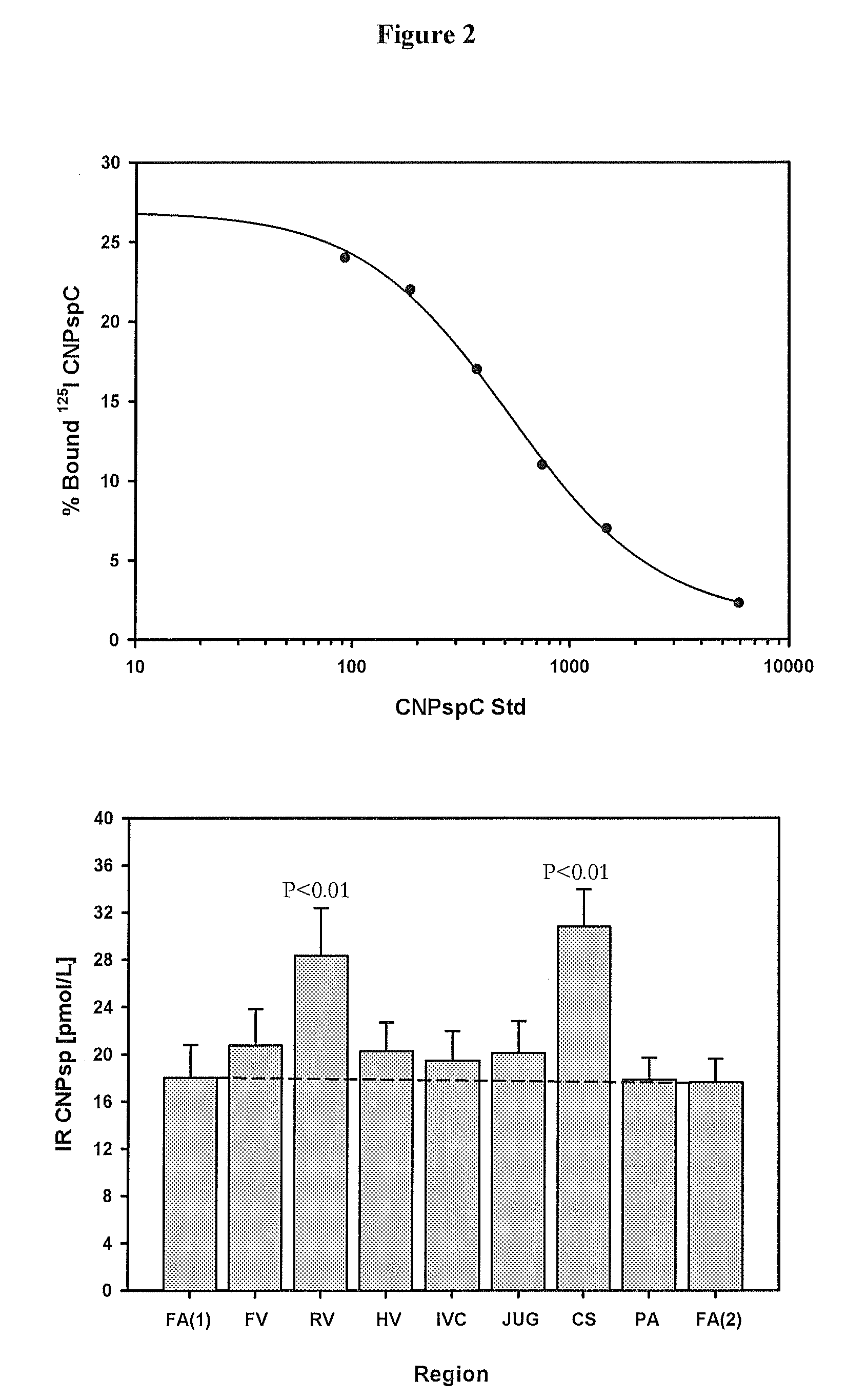
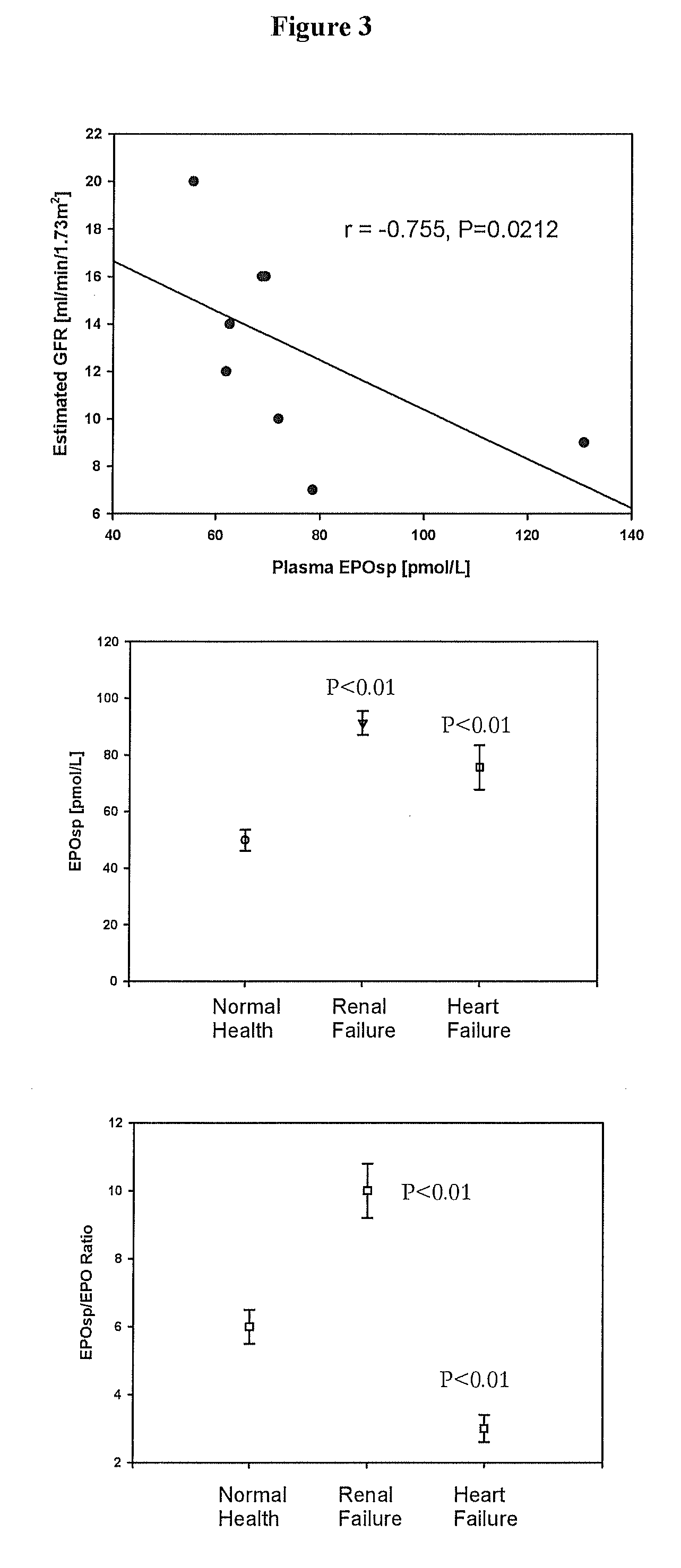
Diagnostics relating to C-type natriuretic and erythropoietin signal peptides and fragments, and kits, uses and applications therefor.
Owner:UPSTREAM MEDICAL TECH LTD
In Vivo production and delivery of erythropoietin or insulinotropin for gene therapy
InactiveUS6846676B2Cost of treatment can be reducedAccurate of functionPeptide/protein ingredientsGenetic material ingredientsHeterologousErythroid cell
The invention provides primary and secondary cells that are transfected with a nucleic acid molecule that encodes erythropoietin, clonal or heterogenous strains of such cells, and methods of producing these cell strains.
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Triazolopyridine compound, and action thereof as prolyl hydroxylase inhibitor or erythropoietin production-inducing agent
The present invention provides a triazolopyridine compound having a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability. The present invention relates to a compound represented by the following formula [I]:wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof, or a solvate thereof, as well as a prolyl hydroxylase inhibitor or erythropoietin production-inducing agent containing the compound. The compound of the present invention shows a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability and is useful as a prophylactic or therapeutic agent for various diseases and pathologies (disorders) caused by decreased production of erythropoietin.
Owner:JAPAN TOBACCO INC
Hypoxia-mediated neurogenesis assay
Methods are described for the production of neurons or neuronal progenitor cells. Multipotent neural stem cells are proliferated in the presence of growth factors and erythropoietin which induces the generation of neuronal progenitor cells. The erythropoietin may be exogenously applied to the multipotent neural stem cells, or alternatively, the cells can be subjected to hypoxic insult which induces the cells to express erythropoietin.
Owner:STEMCELLS CALIFORNIA
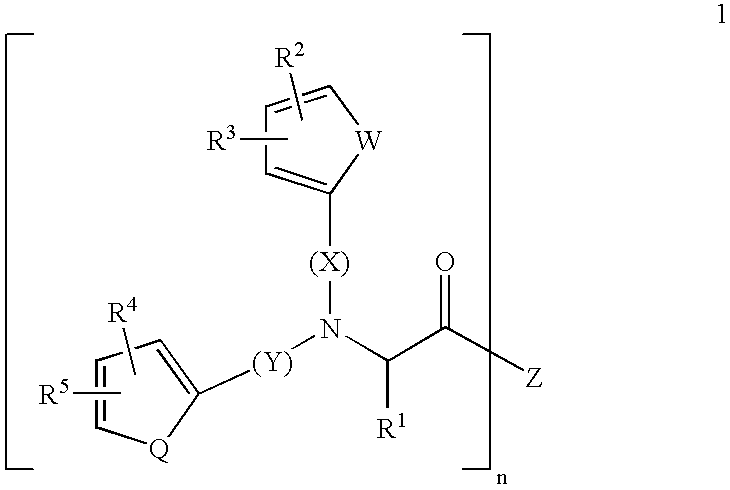
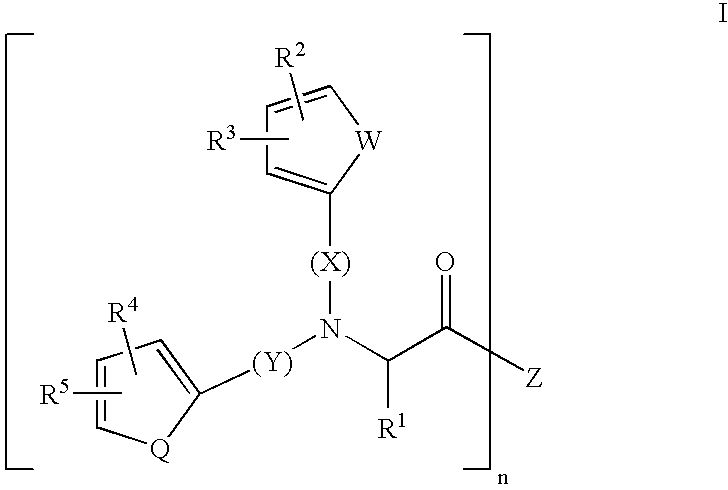
Substituted amino acids as erythropoietin mimetics
InactiveUS20060247274A1Easy to managePotential for oral activityBiocideOrganic chemistrySubstituted Amino AcidErythropoietin receptor
This invention relates to a series of substitituted amino acids of Formula I pharmaceutical compositions containing them and intermediates used in their manufacture. The compounds of the invention are small molecules which bind to the erythropoietin receptor and compete with the natural ligand for binding to this receptor.
Owner:ORTHO MCNEIL PHARM INC
Methods for the production of cytoprotective asialo-erythropoietin in plants and its purification from plant tissues
PendingUS20150119325A1Improve the level ofEasy to producePeptide/protein ingredientsImmunoglobulinsNucleotidePlant cell
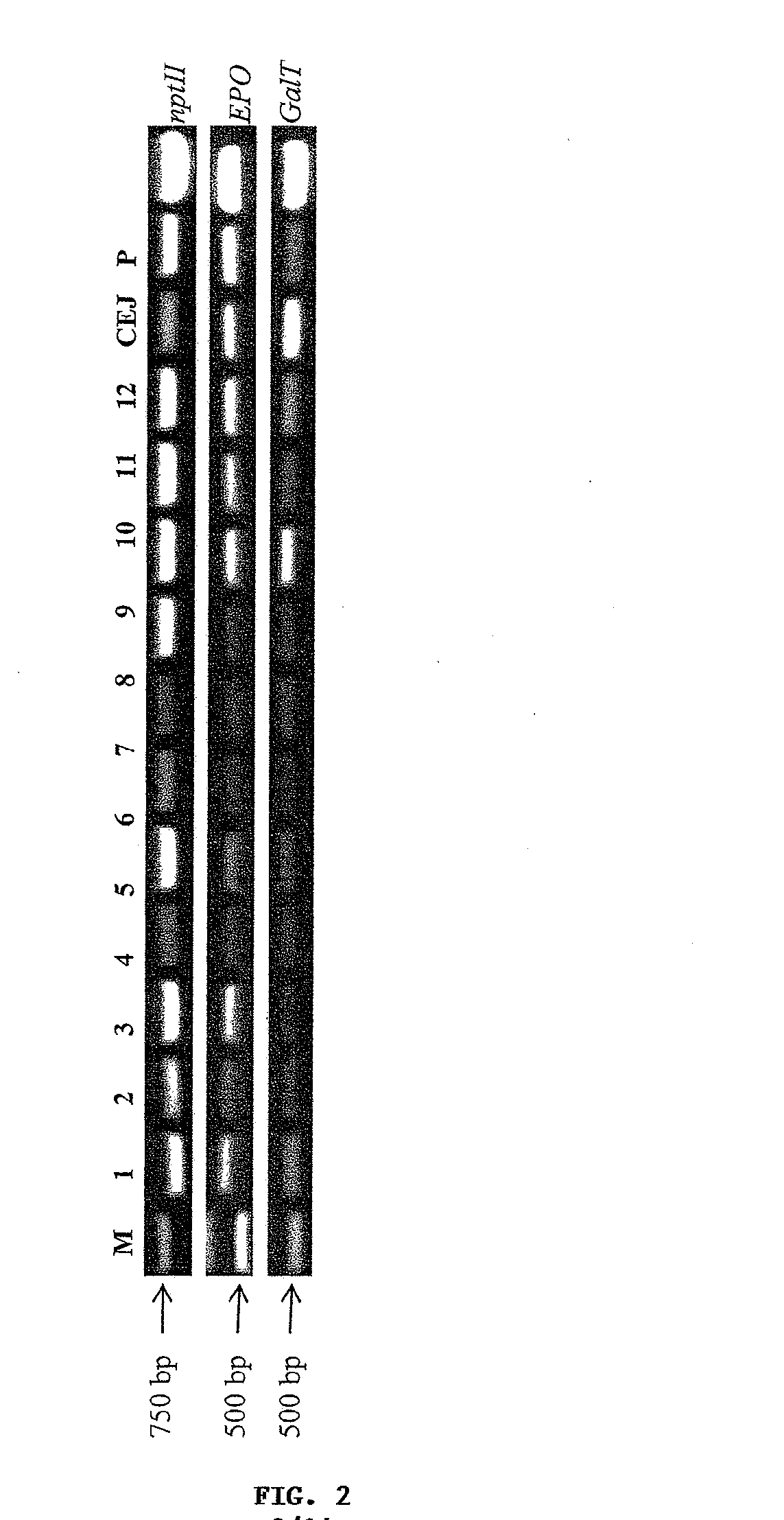
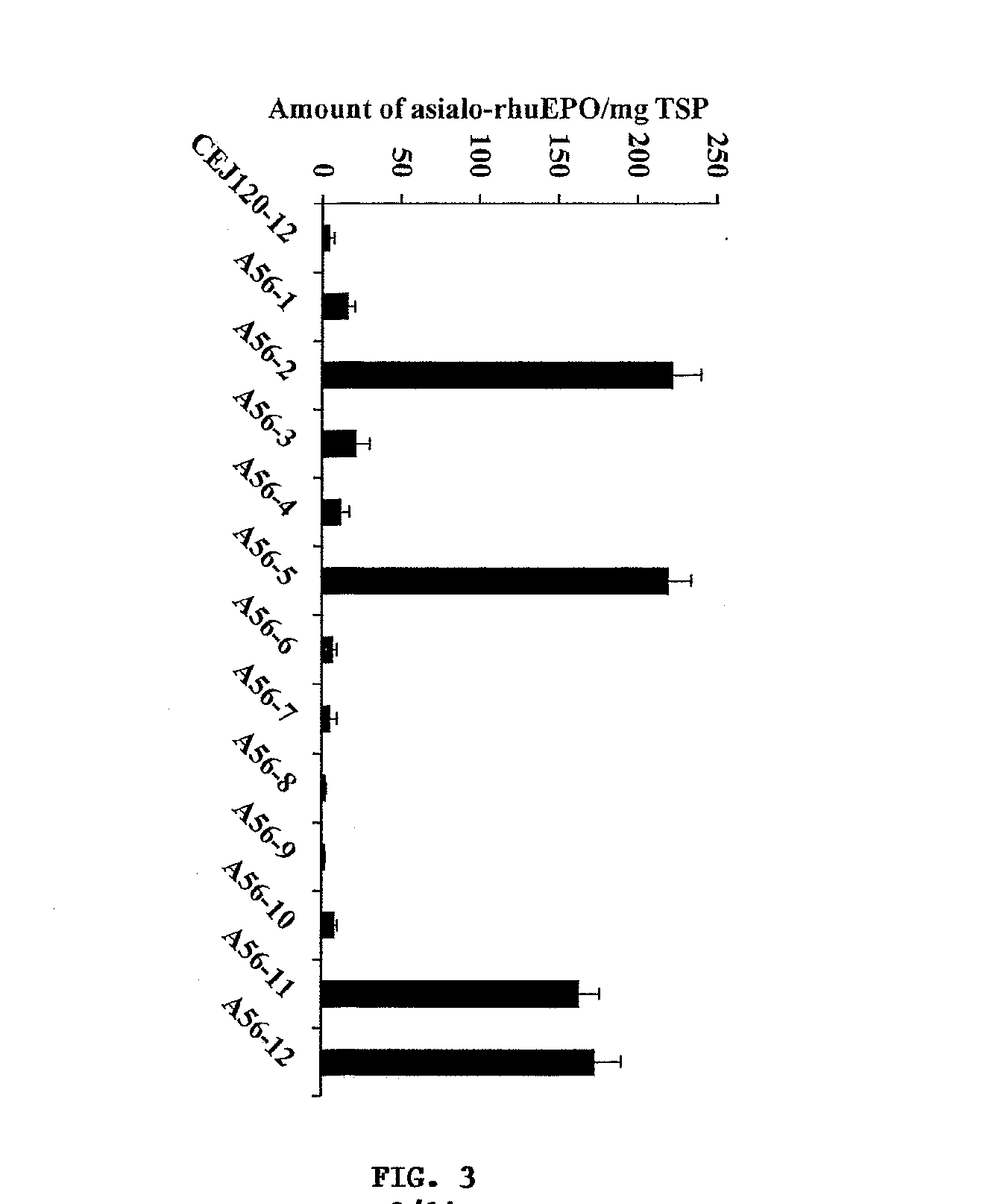
The present invention provides methods for the high-level production of recombinant human erythropoietin (rhuEPO) derivative, asialoerythropoietin (asialo-rhuEPO), in plants. The methods for producing asialo-rhuEPO comprise making a plant or at least one plant cell that comprises a promoter that drives expression in a plant cell operably linked to a polynucleotide encoding a human erythropoieting fusion protein and a promoter that drives expression in a plant cell operably linked to a polynucleotide encoding N-glycosylation modification enzyme, particularly a mammalian β1,4-galactosyltransferase. The present invention further provides plants, plant cells, and seeds that have been genetically modified to produce high levels of asialo-rhuEPO. Additionally, provided are methods for purifying asialo-rhuEPO from plant tissues. Such methods comprise removing chlorophyll and / or RuBisCO protein from an aqueous extract of plant tissue comprising asialo-rhuEPO, binding the asialo-rhuEPO in the extract to an immune affinity column, and eluting the bound asialo-rhuEPO from immune affinity column.
Owner:NORTH CAROLINA CENTRAL UNIVERSITY
Long lasting drug formulations
InactiveUS9127084B2Improve the level ofBiocidePeptide/protein ingredientsNucleic acid sequencingRegulatory sequence
The present invention is directed to long-lasting erythropoietin therapeutic formulations and their methods of use wherein the formulation comprises a genetically modified micro-organ that comprises a vector which comprises a nucleic acid sequence operably linked to one or more regulatory sequences, wherein the nucleic acid sequence encodes erythropoietin.
Owner:MEDGENICS MEDICAL ISRAEL
Novel polynucleotide molecules for enhanced gene expression
InactiveUS20120129770A1Enhance protein expressionPeptide/protein ingredientsVertebrate antigen ingredientsNucleotideIntein
Disclosed herein is an isolated polynucleotide molecule encoding protein such as erythropoietin or structural variants, comprising an expression regulating nucleotide sequence operatively linked to a nucleotide sequence(s) encoding said proteins. The said expression regulatory region comprises of Exon A and at least proximal region of Intron A of Major Immediate Early Region of human Cytomegalovirus or its functional variants which when transfected into host cells results in many fold increase in expression of the protein.
Owner:IPCA LAB LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com