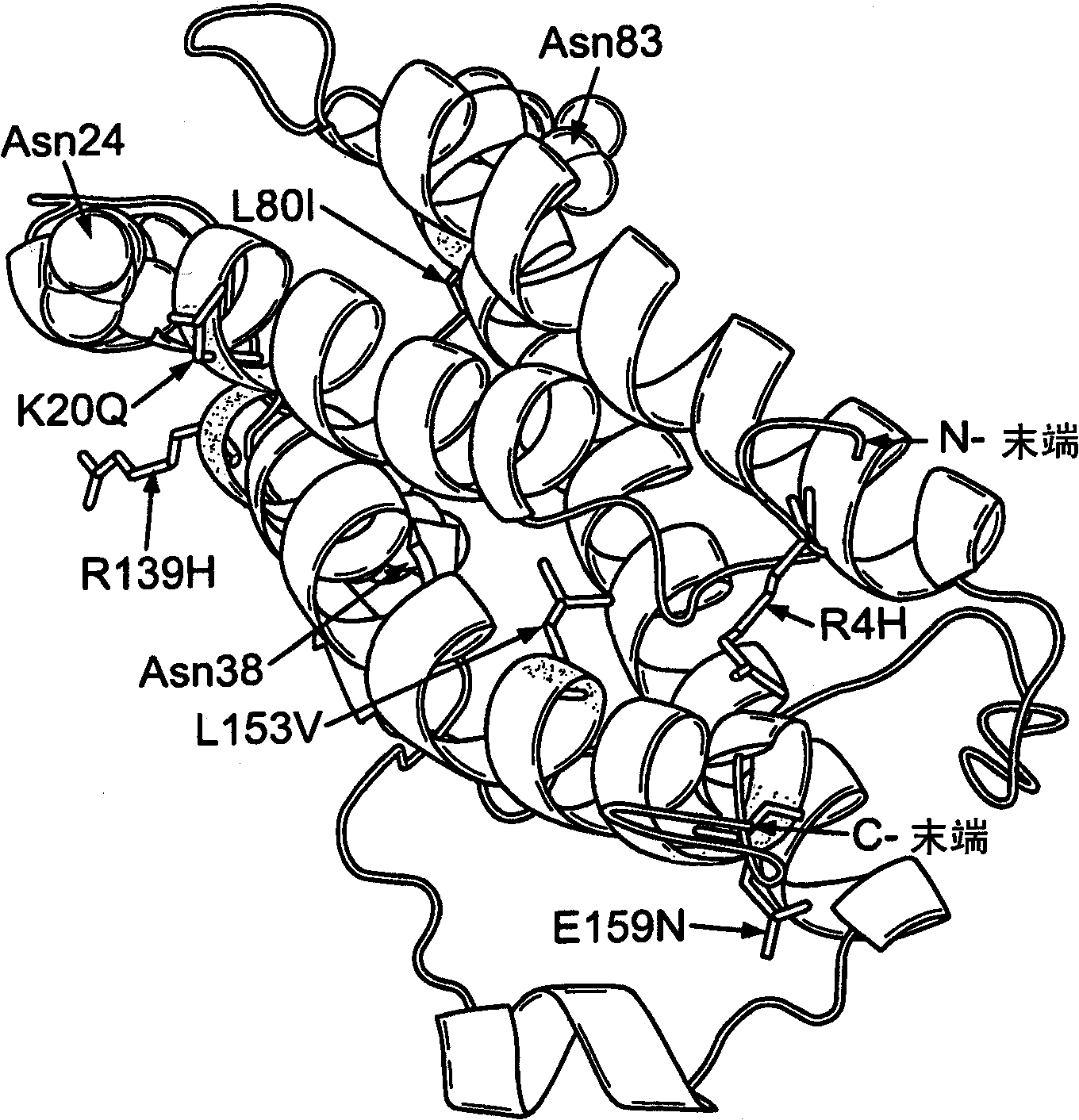
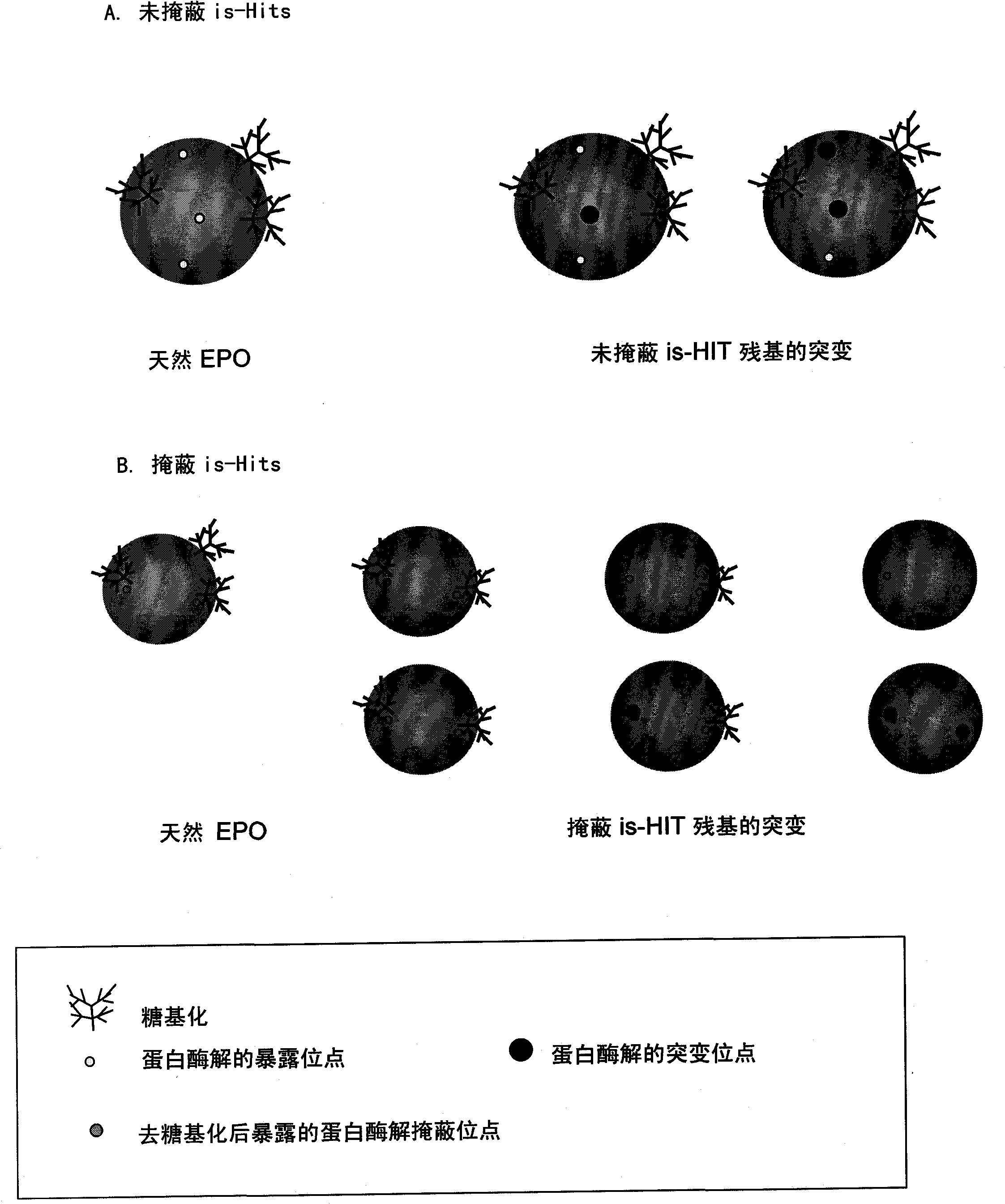
Modified erythropoietin (epo)polypeptides that exhibit increased protease resistance and pharmaceutical compositions thereof
A technology of erythropoietin and composition, which is applied in the direction of erythropoietin, drug combination, peptide/protein composition, etc., can solve the problems of highly variable, difficult, and expensive preparation of glycosylated therapeutic protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0720] Cloning and production of EPO mutants
[0721] 1. Cloning of cDNA encoding EPO and inserting into mammalian expression vector
[0722] Use the following primers from GeneStorm using standard techniques known in the art Human clone IDRG001720 (from the ResGen ORF positive collection-catalog number #H-K1000, Invitrogen; the sequence is carried in the mammalian expression plasmid pcDNA3.1 / GS, Invitrogen, SEQ ID NO: 230) by polymerase chain reaction (PCR) ) Amplify the nucleotide sequence including the coding sequence of human erythropoiesis factor (SEQ ID NO: 229):
[0723] EPO BamHI forward primer:
[0724] 5'-GGGAATTCCATATGGGGGTGCACGAATGTCCTGCCTGG-3' (SEQ ID NO: 231) and
[0725] EPO NdeI reverse primer:
[0726] 5'-CGGGATCCTCATCTGTCCCCTGTCCTGCAGGCCTCCC-3' (SEQ ID NO:232).
[0727] The amplified sequence human erythropoietin cDNA sequence was cloned into pTOPO-TA vector (Invitrogen) to generate plasmid pTOPO-TA-hEPO. The sequence of the EPO cDNA was detected by automated DNA sequ...
Embodiment 2
[0742] Production and yield determination of natural and modified human EPO polypeptides (proteins) in mammalian cells
[0743] Chinese hamster ovary (CHO) cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum (FCS). On the day before transfection, take 5x10 per well 5 The density of the cells, in DMEM containing 10% FCS (without antibiotics), spread the CHO cells in a 6-well plate at 37° C. in a humidified atmosphere containing 7% CO2 for the next few days after transfection Achieve 50-90% confluence every day.
[0744] The Perfectin reagent (Ozyme) was used to transfect CHO cells with 2 μg of EPO mutant DNA according to the manufacturer's instructions. After transfection, plate the cells in Opti-MEM Reduced serum medium (Invitrogen) at 37°C containing 7% CO 2 The ingredients are incubated for 4 hours in a humid atmosphere. After incubation, the transfection medium was replaced with fresh 1 ml DMEM medium containing 1% FCS. After 96 hours, the ...
Embodiment 3
[0747] Measure the specific activity of human EPO by TF-1 proliferation test
[0748] Proliferation test was performed on each aliquot sampled at different time points of the protease degradation kinetics in the human erythroleukemia cell line TF-1 bioassay to determine the residual proliferation activity (EC 50 ).
[0749] In RPMI 1640 medium (Invitrogen) supplemented with 10% FCS, 2mM L-glutamine, and 2ng / ml human recombinant GM-CSF, at 37°C and containing 7% CO 2 / 95% air content in a humid atmosphere, under T175 (175cm 2 ) Maintain the TF-1 cell line in a polystyrene tissue culture flask and divide twice a week. Twenty-four hours before use in the proliferation test, the cells were washed twice in ice-cold PBS, and resuspended in GM-CSF-free RPMI medium supplemented with 2mM glutamine and 10% FCS for 16 hours .
[0750] Divide TF1 cells at 4x10 per well 4 The cells were plated in a 96-well plate in 70 μl of GM-CSF-free RPMI medium supplemented with 2mM glutamine and 10% FCS. Ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com