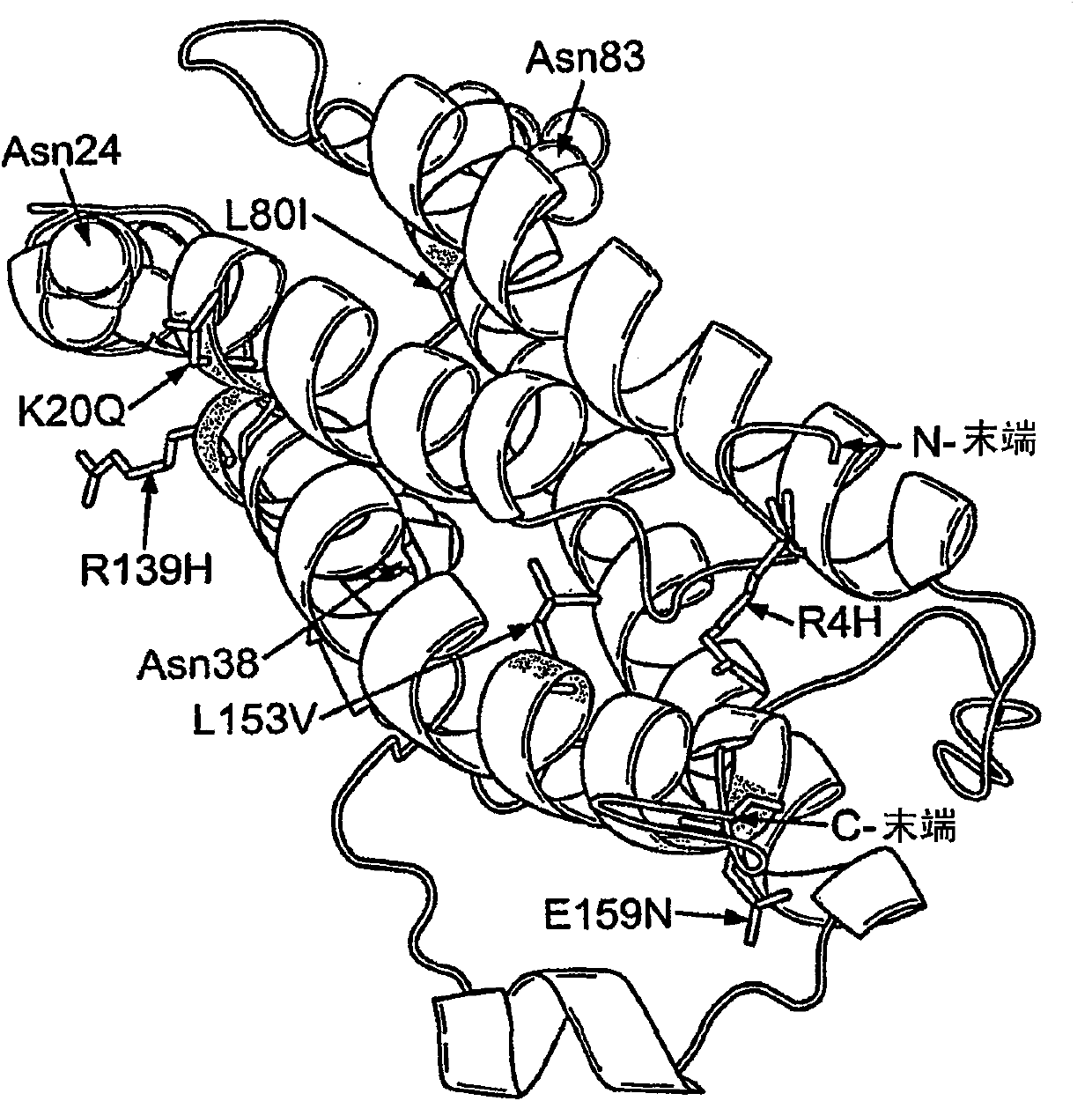
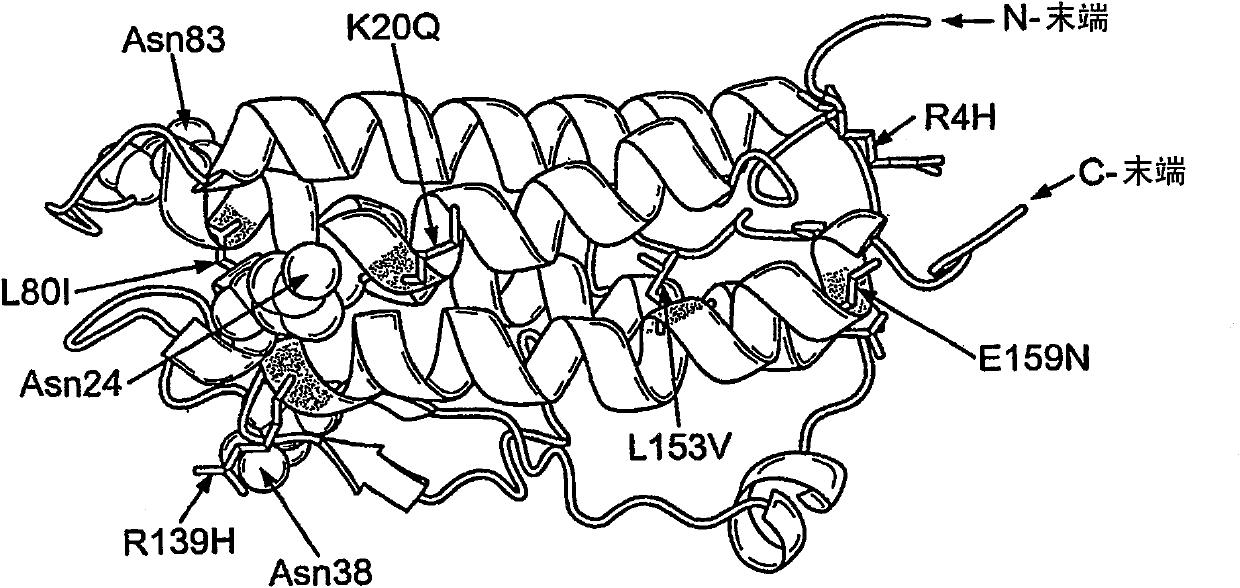
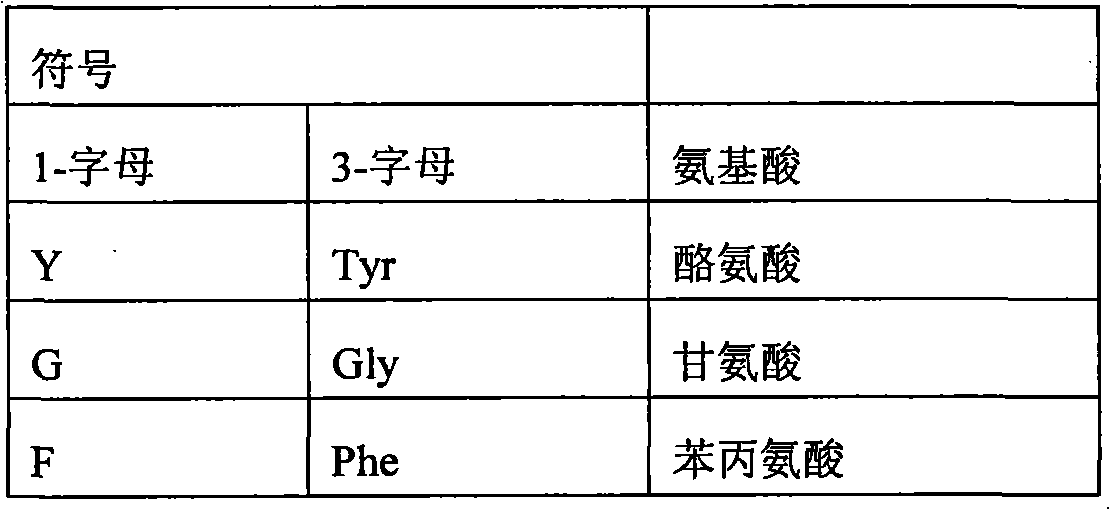
Modified erythropoietin polypeptides and uses thereof for treatment
A therapeutic, unmodified technology, applied in the field of modified erythropoietin polypeptides and their therapeutic uses, can solve the problems of difficult, highly variable, and expensive preparation of glycosylated therapeutic proteins, and achieve increased protein half Lifespan, effect of increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0696] Cloning and production of EPO mutants
[0697] 1. Cloning of cDNA encoding EPO and insertion into mammalian expression vector
[0698] Using the following primers using standard techniques known in the art from Human clone IDRG001720 (from the ResGenORF expression positive pool - catalog #H-K1000, Invitrogen; sequence carried in mammalian expression plasmid pcDNA3.1 / GS, Invitrogen, SEQ ID NO: 230) was amplified by polymerase chain reaction (PCR) Nucleotide sequence including the coding sequence of human erythropoietin (SEQ ID NO: 229):
[0699] EPOBamHI forward primer: 5'-GGGAATTCCATATGGGGGTGCACGAATGTCCTGCCTGG-3' (SEQ ID NO: 231) and
[0700] EPONdeI reverse primer: 5'-CGGGATCCTCATCTGTCCCCTGTCCTGCAGGCCTCCC-3' (SEQ ID NO: 232).
[0701] The amplified sequence human erythropoietin cDNA sequence was cloned into pTOPO-TA vector (Invitrogen) to generate plasmid pTOPO-TA-hEPO. The sequence of the EPO cDNA was detected by automated DNA sequencing. The pTOPO-TA-hEPO plasm...
Embodiment 2
[0716] Production and Yield Determination of Natural and Modified Human EPO Polypeptides (Proteins) in Mammalian Cells
[0717] Chinese hamster ovary (CHO) cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum (FCS). The day before transfection, 5×10 per well 5 Density of cells in DMEM containing 10% FCS (without antibiotics) at 37°C in 7% CO 2 The CHO cells were plated in 6-well plates in a humidified atmosphere of components to achieve 50-90% confluency a few days after transfection.
[0718] CHO cells were transfected with 2 μg of EPO mutant DNA using Perfectin reagent (Ozyme) according to the manufacturer's instructions. After transfection the cells were plated in Reduced serum medium (Invitrogen) at 37°C in 7% CO 2 Components were incubated for 4 hours in a humid atmosphere. After incubation, the transfection medium was replaced with fresh 1 ml DMEM medium containing 1% FCS. Cell supernatants were collected after 96 hours, aliquoted...
Embodiment 3
[0721] Determination of specific activity of human EPO by TF-1 proliferation assay
[0722] Proliferation assays were performed on aliquots sampled at different time points of protease degradation kinetics in the human erythroleukemia cell line TF-1 bioassay to determine the residual proliferative activity (EC 50 ).
[0723] In RPMI1640 medium (Invitrogen) supplemented with 10% FCS, 2mML-glutamine and 2ng / ml human recombinant GM-CSF, at 37°C, in 7% CO 2 / 95% air composition in a humid atmosphere, at T175 (175cm 2 ) polystyrene tissue culture flasks and split twice weekly. Twenty-four hours before use in proliferation assays, cells were washed twice in ice-cold PBS and resuspended in GM-CSF-free RPMI medium supplemented with 2 mM glutamine and 10% FCS for 16 hours .
[0724] TF1 cells were divided into 4 × 10 per well 4 Cells were plated in 96-well plates in 70 [mu]l of GM-CSF-free RPMI medium supplemented with 2 mM glutamine and 10% FCS. Aliquots were two-fold serially d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com