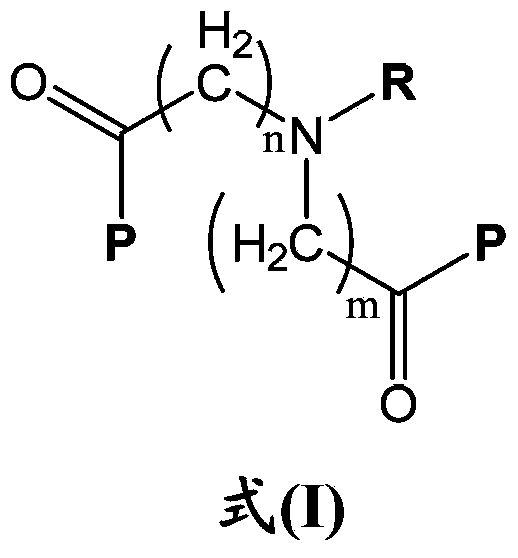
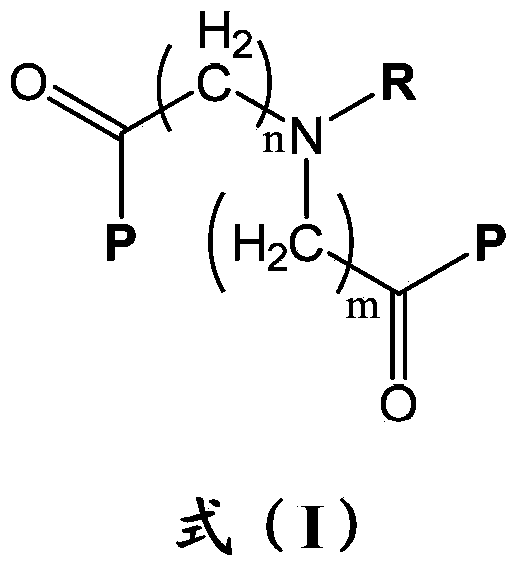
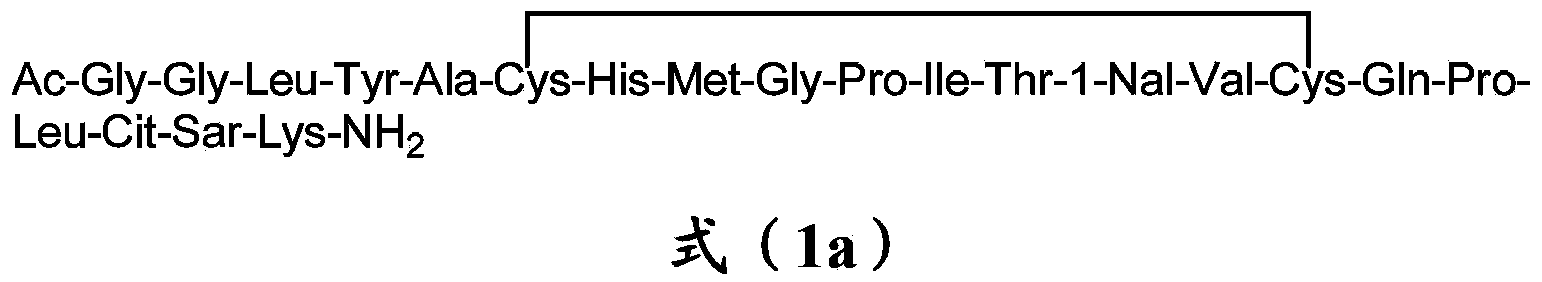Erythropoietin mimetic peptide chemical dimer and use thereof
An erythropoietin and peptide-mimicking technology, applied in erythropoietin, extracellular fluid diseases, chemical instruments and methods, etc., can solve problems such as poor in vivo stability and inability to meet the conditions for ready-made medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0127] Embodiment 2: the synthesis of SEQ-02
[0128]
[0129] a) Synthesis of N-tert-butoxyyl-β-alanyl-iminodiacetic acid hydroxysuccinimide ester (2a) become
[0130]
[0131] Put 4.03g (25mmol) of dimethyl iminodiacetate in a 250ml eggplant-shaped bottle, add 100ml of dry dichloromethane, add 4.7g (25mmol) of BOC-β-Ala-OH, and dissolve 4.1g (30mmol) of HOBt in 10ml of DMF Add the reaction bottle into the reaction bottle, stir under the ice bath, finally add 5.82g (30mmol) EDC, return to the temperature naturally, and stir overnight. Concentrate under reduced pressure, add ethyl acetate to dissolve the residue, in a separatory funnel, successively saturated NaCl, 10% HCl aqueous solution, saturated NaCl, saturated NaHCO 3 , saturated NaCl wash, organic layer anhydrous Na 2 SO 4 dry. After filtration and concentration to obtain an oily substance, the oil pump was drained to obtain 7.07 g of a foamy solid, with a yield of 84%.
[0132]Weigh 5g (15mmol) of the ...
experiment example 1
[0136] Biological experiment example 1: EPO receptor-mediated cell proliferation activity evaluation
[0137] The method for detecting EPO activity in vitro is mainly based on the study of EPO-induced proliferation and / or differentiation of EPO-sensitive cells. TF-1 cells (purchased from GenScript Corporation) are such cells. The TF-1 cell line was first isolated from human erythrocytic leukemia patients, and the cells highly express EPOR. The proliferation of TF-1 cells depends on granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin 3 (IL-3). EPO can also induce the proliferation of TF-1 cells, and has been used as a generally accepted method for in vitro detection of EPO activity (T Kitamura, et al.Blood.1989, 73:375-380.S Chretien, et al.The EMBO J .1996, 15:4174-4181). The proliferative activity of EPO receptor-mediated erythropoietin mimetic peptide was determined at the cellular level. The results are shown in Table 1.
[0138] Table 1. Cell pr...
experiment example 2
[0141] Biological experiment example 2: evaluation of red blood cell activity in normal mice
[0142] Animals: mice (KM, male, 18-22g, Experimental Animal Center, Academy of Military Medical Sciences);
[0143] Drug preparation: the drug is ready-to-use, with 0.1% BSA saline as the vehicle.
[0144] Automatic blood analyzer (French YODER company).
[0145] Test method: Adult male mice were randomly divided into 5 groups, about 10 in each group. Animals were administered continuously for 7 days, 1 time / d, subcutaneous injection (sc), and blood was collected to measure various indicators at the end of the test (entrusted to 307 Hospital for testing). Use rhEPO (recombinant EPO, Huanerbo, 5000IU / mL, Sihuan Biopharmaceutical Co., Ltd.) as a positive control.
[0146] Experimental results: see Table 2.
[0147] Table 2 Effects of Compounds on the Production of Peripheral Red Blood Cells (RBC), Hemoglobin (Hb) and Reticulocytes (RET)
[0148]
[0149] Note: The vehicle is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com