Methods and compositions for the treatment and management of hemoglobinopathy and anemia
A technology for hemoglobinopathies and anemias, applied in the field of treatment, prevention and/or control of hemoglobinopathies and other anemias, which can solve problems such as unproven efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0161] Specific examples of compounds have the formula:
[0162]
[0163] in:
[0164]One of X and Y is C=O, the other of X and Y is C=O or CH 2 ;
[0165] R 6 is hydrogen, alkyl of 1 to 8 carbon atoms, benzo, chlorine or fluorine;
[0166] R 7 is m-phenylene or p-phenylene or -(C n h 2n )-, wherein the value of n is 0-4;
[0167] R 8 and R 9 Each independently is hydrogen or an alkyl group of 1 to 8 carbon atoms, or R 8 and R 9 Linked together is tetramethylene, pentamethylene, hexamethylene, or -CH 2 CH 2 x 1 CH 2 CH 2 -, where X 1 is -O-, -S-, or -NH-; and
[0168] R 10 is hydrogen, an alkyl group of 1 to 8 carbon atoms, or a phenyl group.
[0169] Preferred immunomodulatory compounds of the invention are 4-(amino)-2-(2,6-dioxo(3-piperidinyl))-isoindoline-1,3-dione and 3-(4- Amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione. This compound can be obtained by standard synthetic methods (see, eg, US Patent 5,635,517, which is incorporated here...
Embodiment 1
[0309] 6.1. Example 1: Differentiation of bone marrow-derived CD34+ hematopoietic progenitor cells into dendritic cells demonstrates upregulation of erythroid-specific genes
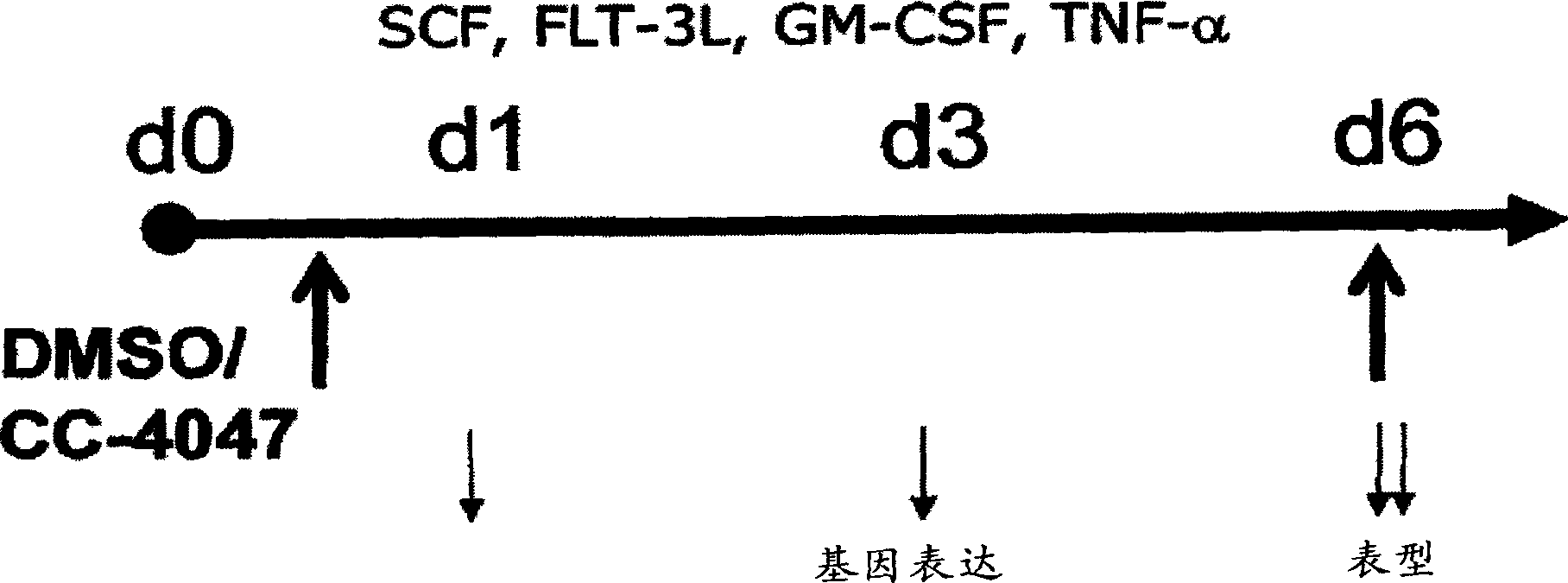
[0310] BM-CD34 + Cells were obtained from Cambrex (East Rutherford, NJ) and cultured in BIT 95000 (StemCell Teclmologies, UK) in Iscove's MDM for 6 days. In order to study the effect of 4-(amino)-2-(2,6-dioxo(3-piperidinyl))-isoindoline-1,3-dione on dendritic cells, in the presence or CD34+ blasts were cultured for 6 days in the absence of 4-(amino)-2-(2,6-dioxo(3-piperidinyl))-isoindoline-1,3-dione. After 6 days in culture, the phenotypic characteristics of the cells for erythrocyte markers (CD36, CD71, glycophorin A and fetal hemoglobin) were determined by flow cytometry. Gene expression was monitored on days 1, 3, and 6 of CD34+ differentiation by microarray analysis ( figure 1 ).
[0311] RNA purification and microarray analysis. Using RNAeasy (Qiagen) from CD34 +Total RNA is isolated from the ...
Embodiment 2
[0316] 6.2. Example 2: CD34 + cells differentiate into red blood cells
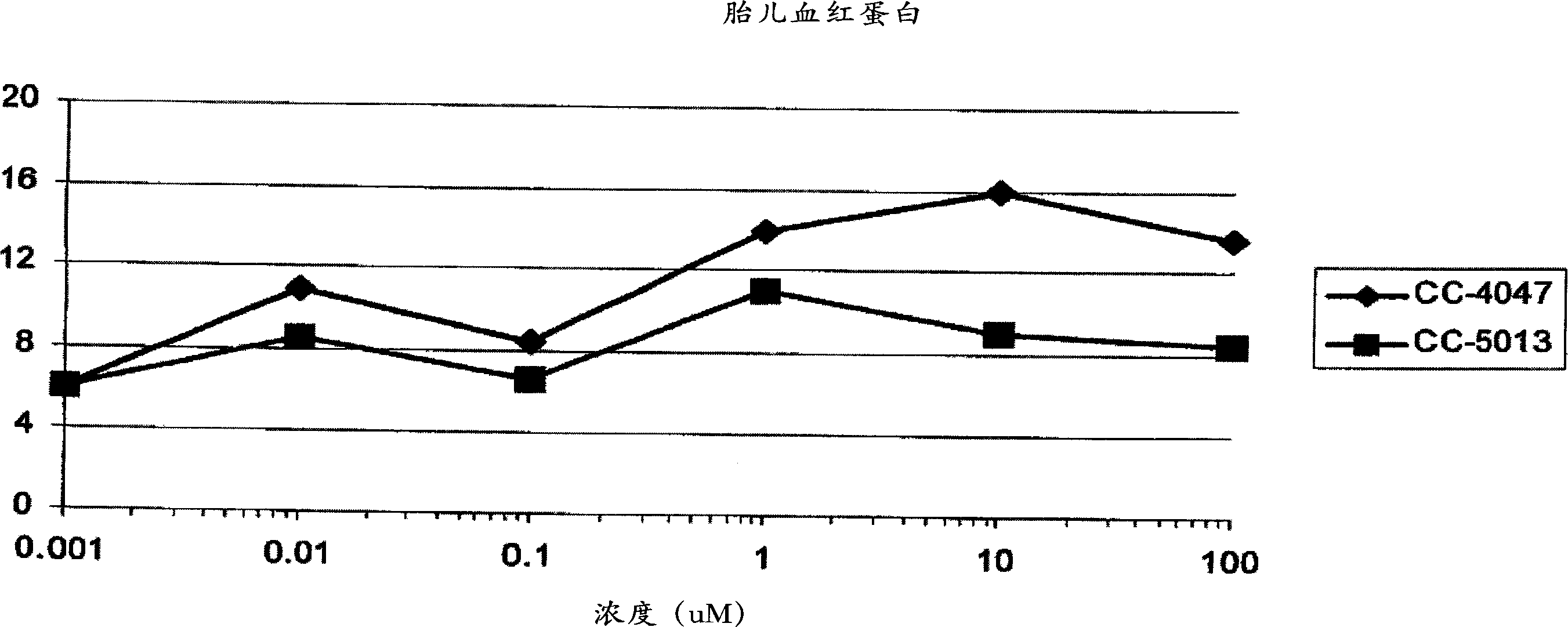
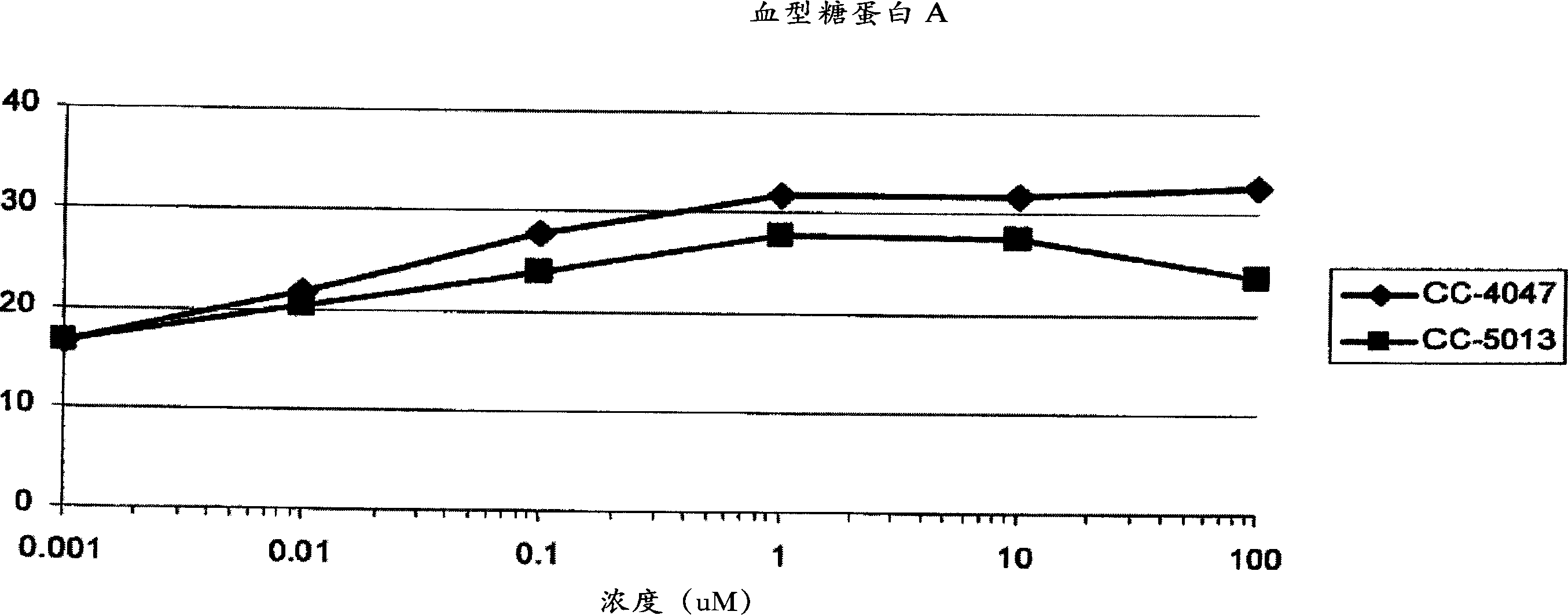
[0317] Differentiation of bone marrow (BM) CD34+ hematopoietic progenitors: BM-CD34+ progenitors were obtained from Cambrex and cultured in Iscove's MDM containing BIT 95000 (serum replacement; StemCell Teclmologies) in the presence of growth factors. During the first 6 days, CD34 + Cells were expanded with SCF (100ng / ml), Flt3-L (100ng / ml) and IL-3 (20ng / ml) and passed through in the presence of SCF (50ng / ml) and Epo (2U or 4U / ml) Differentiate into erythrocytes by culturing for 6 days. To study IMiD TM In the presence or absence of 4-(amino)-2-(2,6-dioxo(3-piperidinyl))-isoindoline-1,3-dione or 3-(4- In the case of amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, differentiate CD34+ progenitor cells for 6 days ( Image 6 ).
[0318] Flow cytometry: Surface antigen expression was analyzed by flow cytometry (FACScan, Coulter) after 6 days of culture. Cells were double-stained on day 6 us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com