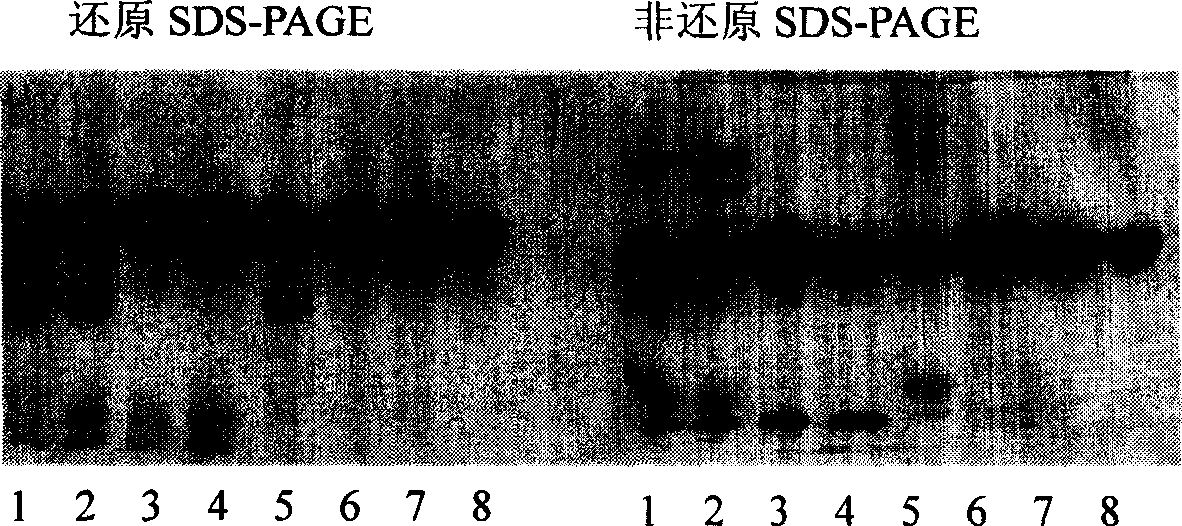
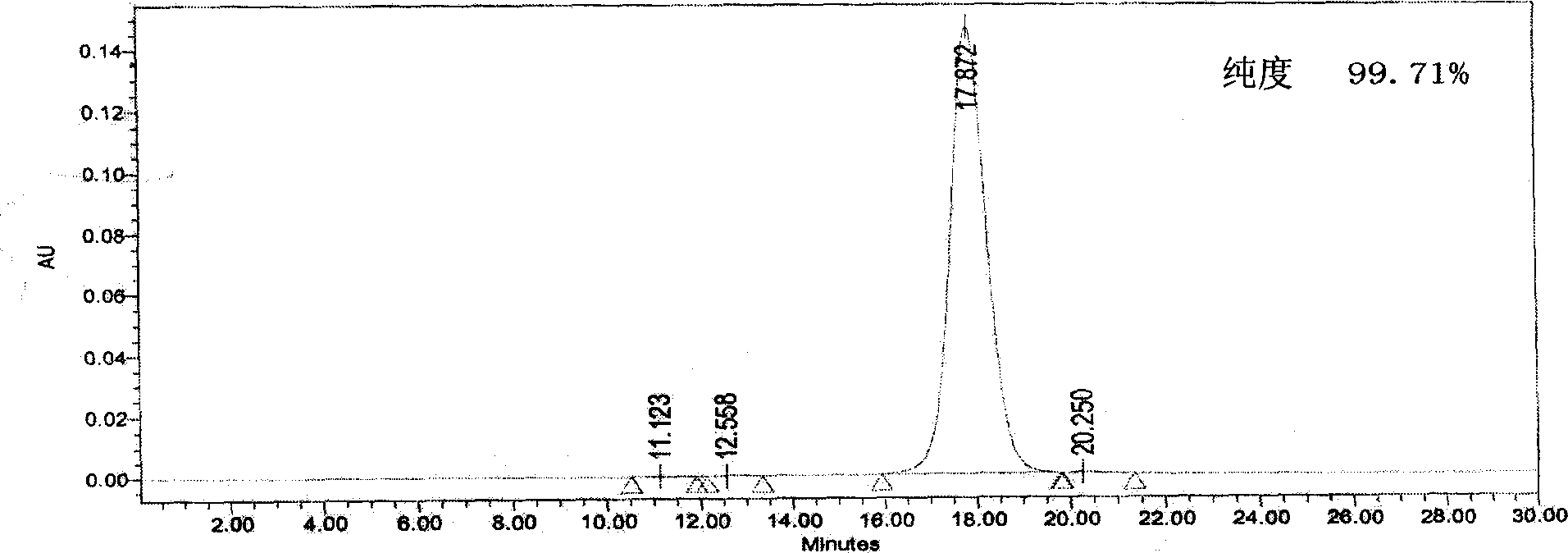
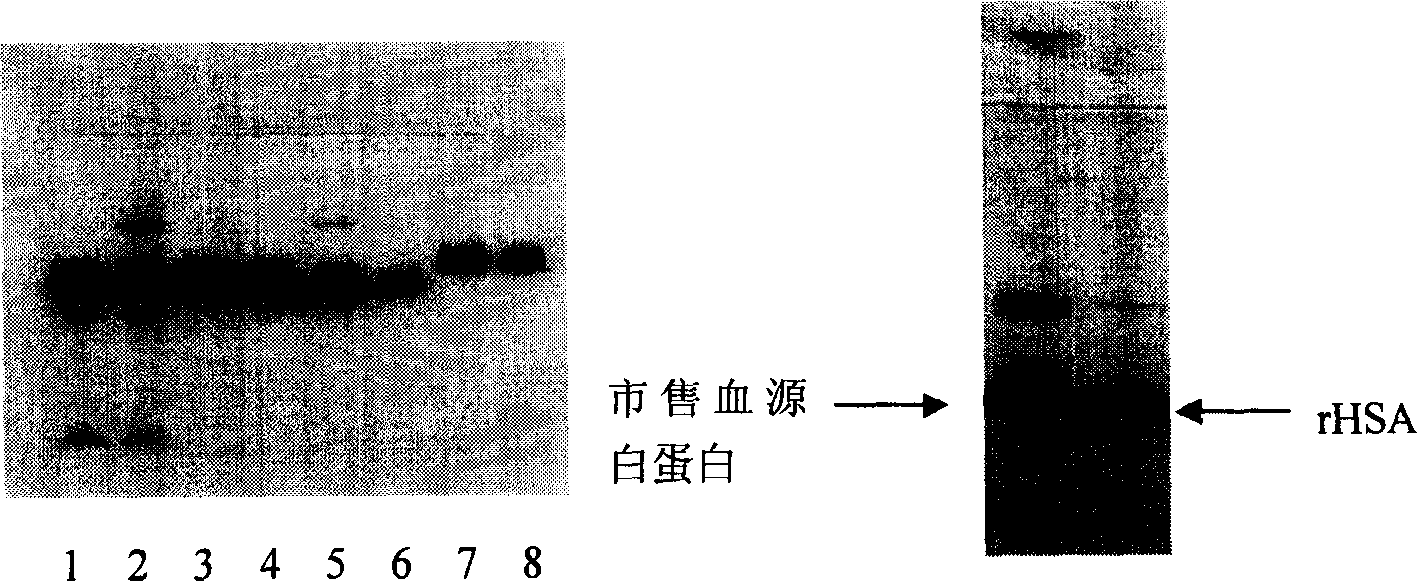
Purification of rHSA
A technology of exchange chromatography and cation exchange, which is applied in the field of protein purification, can solve the problems of high levels of other impurities, and achieve the effects of short experimental period, good application prospects and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The purification process of rHSA of the present invention is as follows:
[0028] In order to reduce the production of pyrogens, the rHSA fermentation broth was filtered with a 0.22um membrane (manufactured by MILLIPORE), and then heated in time to inactivate protease. In order to inhibit the combination of exogenous pigments and target proteins, ensure protein stability, and inhibit the activity of high-temperature-resistant proteases, add decolorization media, stabilizers, and enzyme inhibitors when heating. Decolorization media such as macroporous resin, diatomaceous earth, and activated carbon etc., preferably activated carbon; stabilizers such as palmitic acid, sodium octanoate, etc., enzyme inhibitors such as PMF, EDTA, EGTA, etc., preferably EDTA. In the simultaneous presence of stabilizers, decolorizing media and enzyme inhibitors, adjust the pH of the fermentation supernatant to 6.0-7.0, and then start heating at a temperature of 60-75°C for 10-100 minutes, pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com