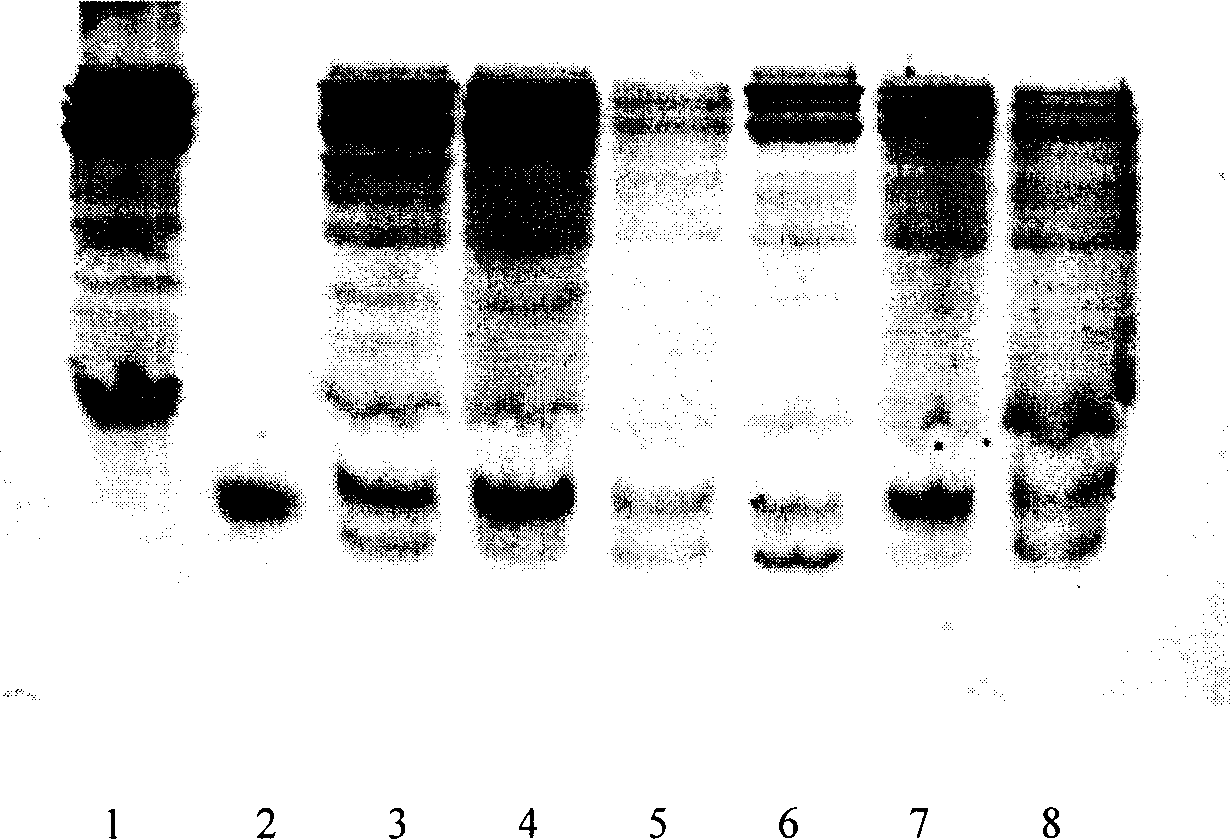Escherichia expression system of secretory expression recombinant human epidermal growth factor
A technology of secretion expression and Escherichia coli, applied in the field of bioengineering, can solve problems such as low content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Chemically synthesized signal peptide phoAspL10 sequence and human epidermal growth factor (hEGF) tandem gene (SEQ ID NO: 2)
[0040] ↓Start codon 5’GAT ACC AAA CAA AGC ACT CTG CTG CTG CTG CTG CTG CTT CTG CTG
[0041] | ← Newly designed signal peptide sequence (the underlined part is CTG CTGACC CCT GTG ACA AAA GCG AAT TCC GAC TCT GAA TGC CCG ten leucines in a row) →|← CTG TCT CAC GAC GGT TAC TGC CTA CAC GAT GGT GTT TGC ATG TAT
[0042] Human epidermal growth factor (hEGF) gene ATC GAA GCT CTG GAC AAA TAC GCG TGC AAC TGT GTT GTT GGT TACATC GGT GAA CGT TGC CAG TAC CGT GAC CTG AAA TGG TGG GAA CTG
[0043]
[0044] When designing and synthesizing the above-mentioned tandem genes, we fully followed the following principles: the codons preferred by Escherichia coli were selected; the contents of AT and GC were close and evenly distributed; the generation of secondary structures in the fragments was avoided.
[0045] At the downstream ...
Embodiment 2
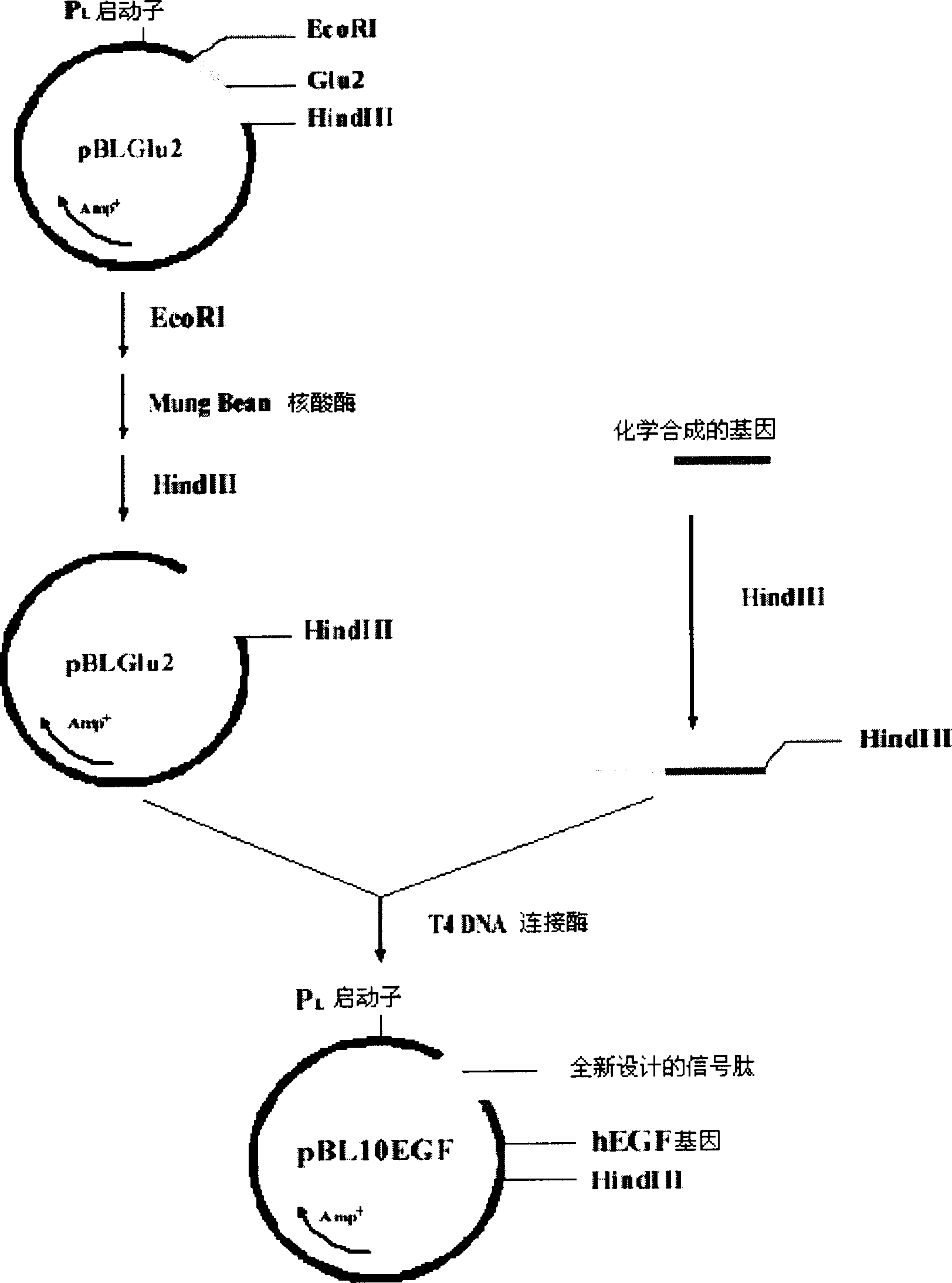
[0046] The construction of embodiment 2 expression plasmid ( figure 1 )
[0047] 2.1 Enzyme digestion treatment of the starting vector pBLGlu2
[0049] Prepare the following reaction mixture:
[0050] 10 μg of plasmid pBLGlu2;
[0051] 20 μl 10×H buffer (TaKaRa);
[0052] 5 μl EcoRI restriction endonuclease (15U / μl, TaKaRa company);
[0053] Make up 200 μl with sterilized double distilled water.
[0054] The above reaction mixture was reacted at 37° C. for 4 hours.
[0055] 2.1.2 Recovery of the vector after digestion
[0056] Add 20μl 3M sodium acetate and 400μl absolute ethanol to the reaction mixture obtained in 2.1.1, mix well, centrifuge at 12,000rpm for 5 minutes, discard the supernatant, then add 400μl 70% ethanol, mix well, 12,000rpm Centrifuge for 2 minutes, discard the supernatant, and vacuum dry.
[0057] 2.1.3 Digestion with Mung Bean Nuclease
[0058] Prepare the following reaction mixture:
[0059] The carrier recovered i...
Embodiment 3
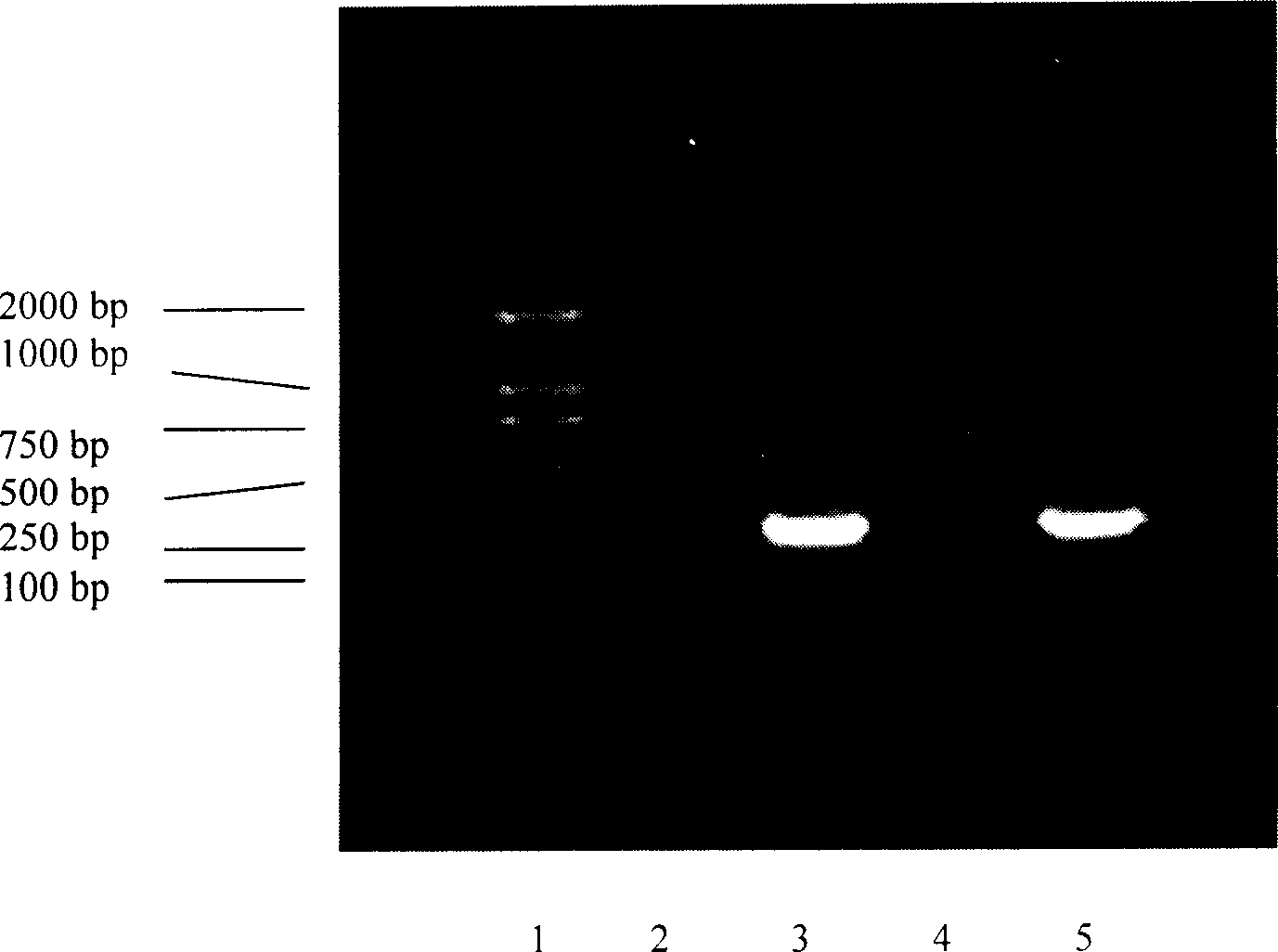
[0115] Example 3 Construction and screening of genetically engineered strains
[0116] 3.1 Construction of genetically engineered strains
[0117] Prepare competent cells of Escherichia coli BL21(DE3) according to the method in 2.4, transform the expression plasmid pBL10EGF into the competent cells of BL21(DE3) according to the method in 2.5, and take 100 μl of LB coated with 100 μg / ml ampicillin Plates (agar content 1.5%) were cultured upside down at 37°C overnight.
[0118] 3.2 Screening of genetic engineering strains
[0119] 3.2.1 Induced expression of genetically engineered strains
[0120] Pick the single colonies obtained in 3.1 and put them into 3 milliliters of LB medium (the concentration of ampicillin is 100 μg / ml), culture overnight at 37°C at 200 rpm, then transfer them into LB medium at a ratio of 1:10, and then After continuing to cultivate for 4 hours, the temperature was raised to 42° C., and the cultivation was terminated after 6 hours. Take 1ml of the cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com