Immunization magnetic separation technology for purifying genetic engineering recombinant interferon
An immunomagnetic separation and recombinant interferon technology, applied in the direction of interferon, cytokines/lymphokines/interferons, animal/human proteins, etc., can solve the problems of increasing the risk of the process, and achieve simplified separation and purification steps, high efficiency The effect of separation and purification, easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
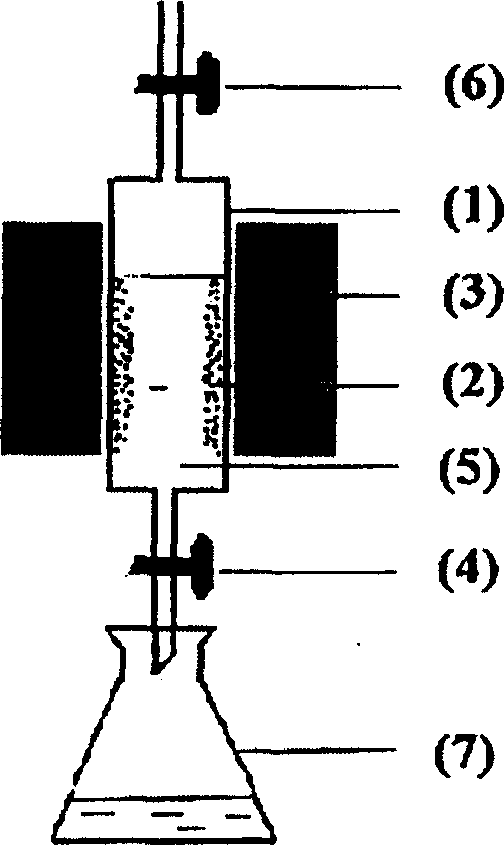
Image
Examples
preparation example Construction
[0013] 1. Preparation of anti-interferon IgG antibody:
[0014] 1) Preparation of Anti-IFN serum:
[0015] Take human interferon (IFN) standard substance, fully mix and emulsify with an equal volume of Freund's complete adjuvant to obtain human interferon (IFN) antigen, take human interferon (IFN) antigen, and treat 2 Japanese white-eared males respectively Rabbits (body weight 2Kg) were intramuscularly and subcutaneously injected at multiple points. The additional injection was prepared by emulsifying the antigen with Freund's incomplete adjuvant, and the booster injection was performed once every two weeks, for a total of three times, with the dose decreasing. Three days after the last immunization, blood was taken from the vein, and the antibody titer was determined by indirect ELISA method. The neutralizing titer of the antibody in the serum was measured to be greater than 1:10000. After that, the abdominal artery was bled, and the whole blood was left to stand for 2 hour...
Embodiment 1
[0036] 1. Preparation of anti-IFNα-2b antibody IgG:
[0037] 1. Preparation of antiserum:
[0038] Take 2 mL of 1 mg / mL IFNα-2b standard substance, mix well with an equal volume of Freund's complete adjuvant and emulsify to obtain IFNα-2b emulsified antigen. Take 2mL of the emulsified antigen of IFNα-2b, and inject it into two rabbits (Japanese big-eared white, male, body weight 2Kg) at multiple points intramuscularly and subcutaneously. The additional injection was prepared by emulsifying the antigen with Freund's incomplete adjuvant, and the booster injection was performed once every two weeks, for a total of three times, with the dose decreasing. Six days after the last immunization, blood was collected from the abdominal artery. The whole blood was left to stand for 2 hours, centrifuged at 3000rpm for 20 minutes, and the serum was collected and inactivated in a water bath at 55°C for 30 minutes.
[0039] 2. Purification of anti-IFNα-2b IgG antibody:
[0040] Take 10 mL ...
Embodiment 2
[0052] 1. Preparation of cellulose immunomagnetic microspheres:
[0053] 1. Preparation of cellulose magnetic microspheres:
[0054] Take 100g of absorbent cotton soaked in 800mL of 19% NaOH solution for 2 hours, squeeze out the lye, and age at 25°C for 3 days; add 50mL of carbon disulfide and mix well, seal and shake at 28°C for 5 hours, then add 750-850mL of 6% NaOH solution to mix Evenly, shake at 28°C for 5 hours to obtain orange-red xanthate viscose. Add 180mL of chlorobenzene, 30mL of carbon tetrachloride and 0.5g of potassium oleate into a 500ml three-neck flask, stir and mix in a water bath at 40°C as the organic phase. Take 70 mL of xanthate viscose solution containing 1 gram of magnetic fluid, add it to the organic phase, disperse at 3000 rpm for 30 minutes, then rapidly raise the temperature to 90° C., and continue to react for 2 hours to obtain cellulose magnetic microspheres. Rinse with ether and distilled water respectively, sieve and screen the cellulose magne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com