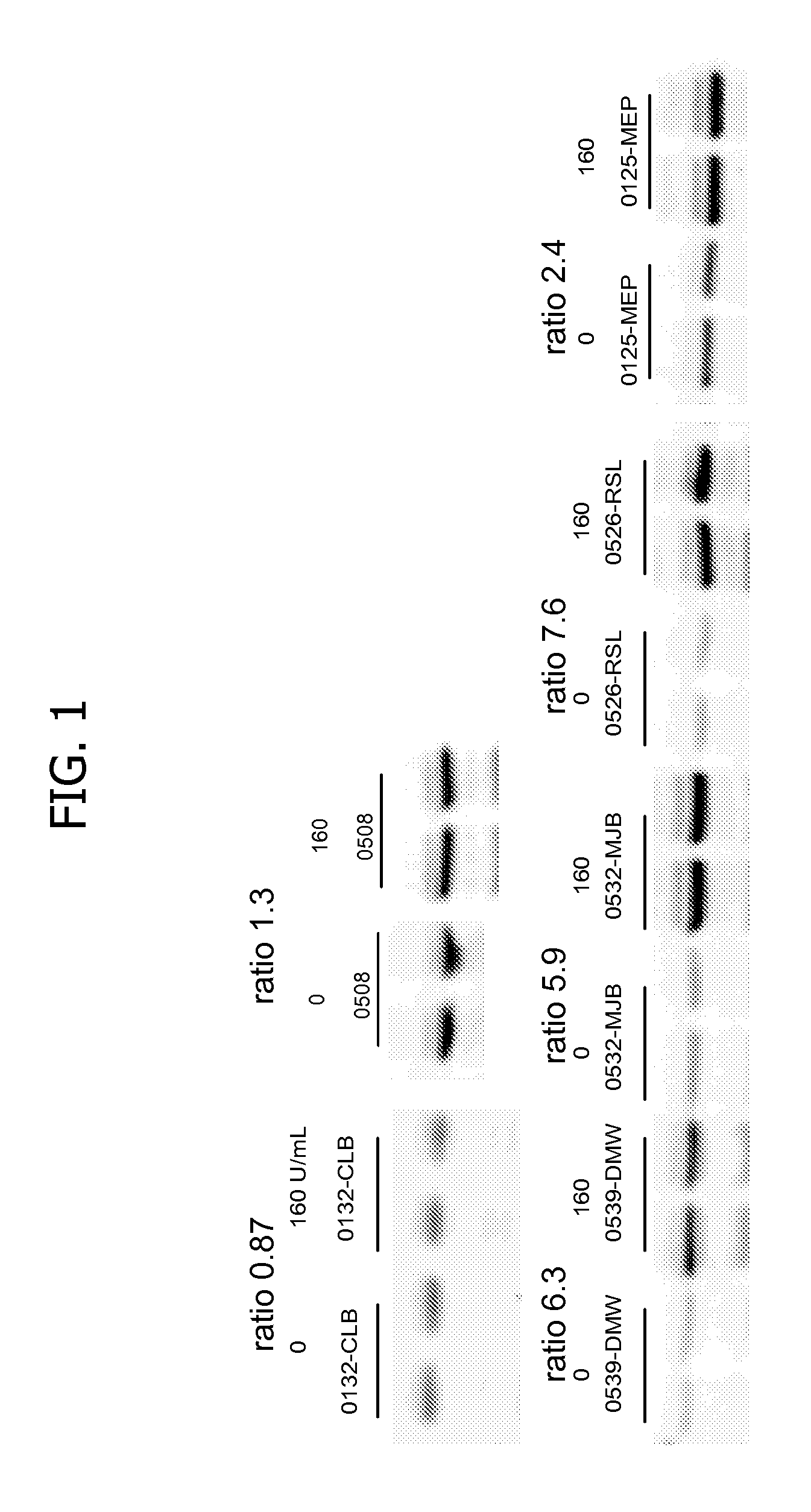
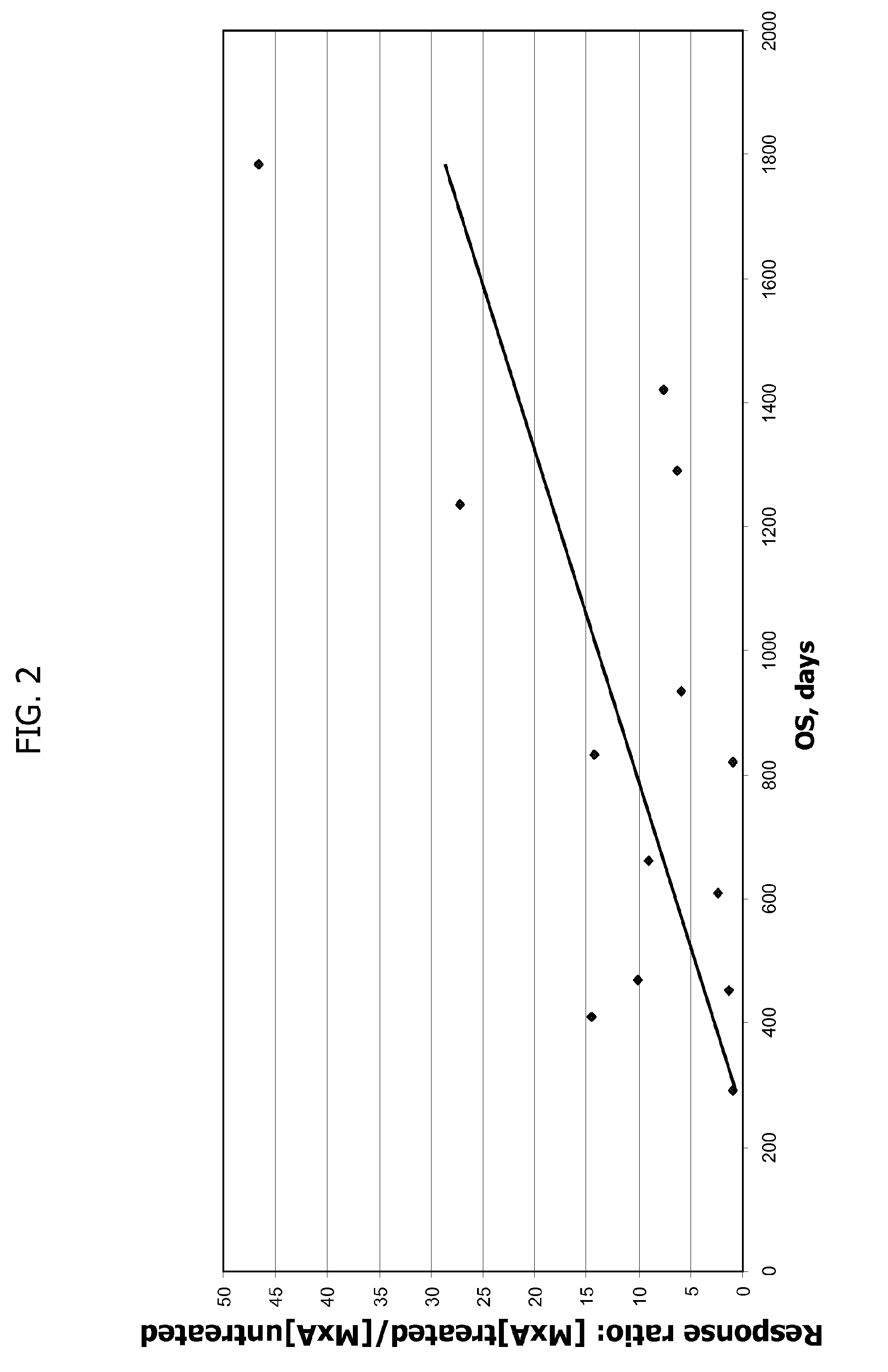
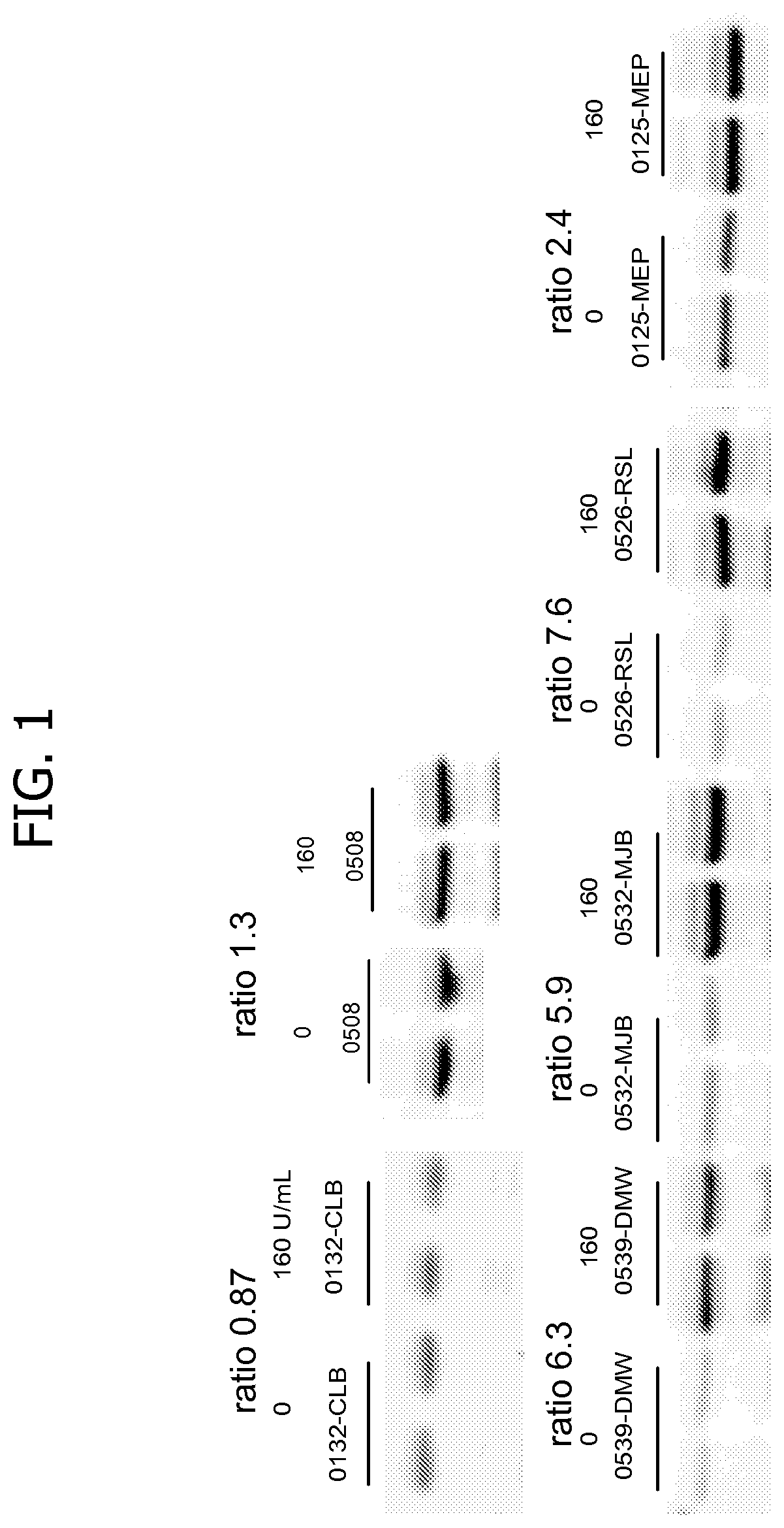
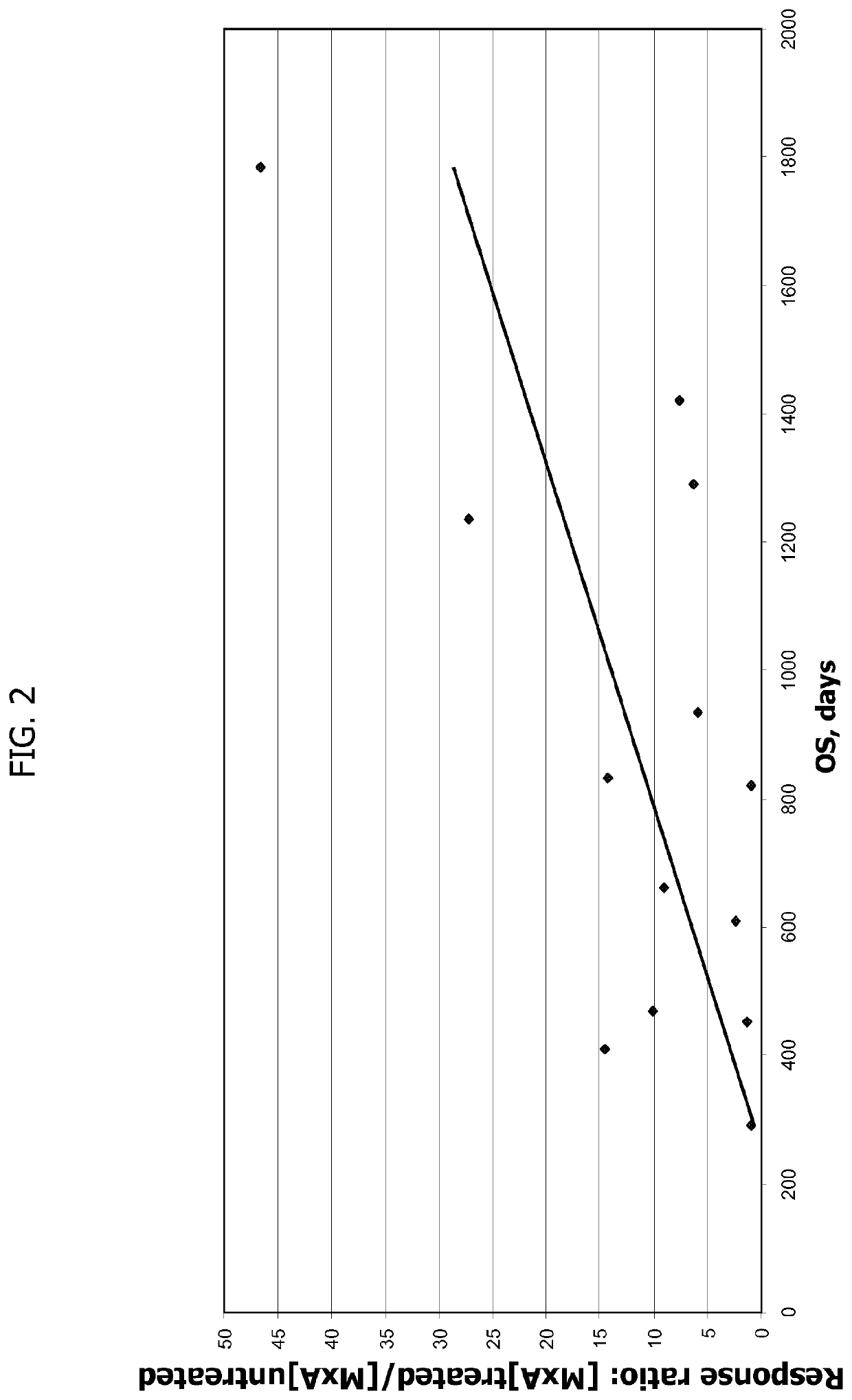
Patents
Literature
46 results about "Type 1 interferon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
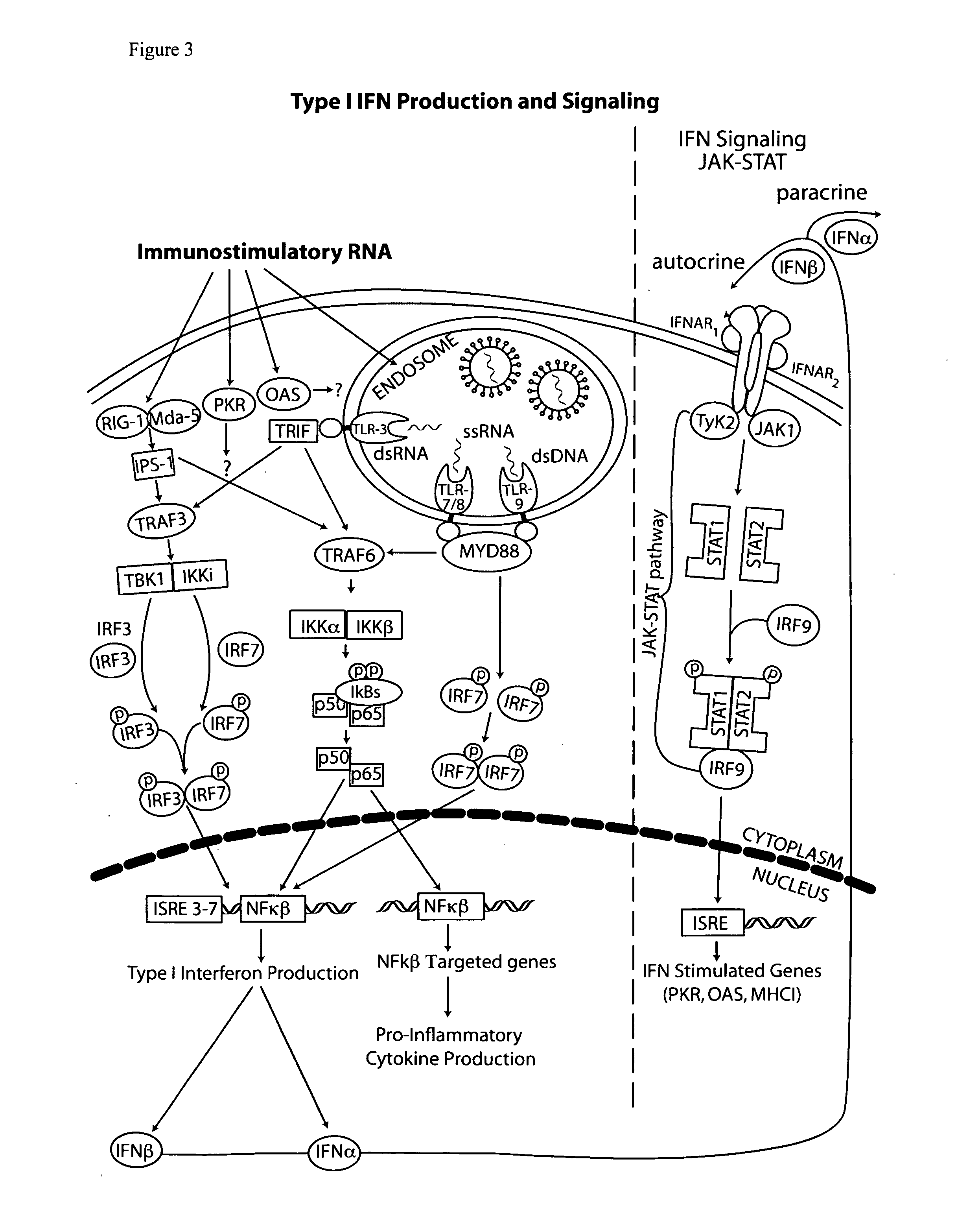
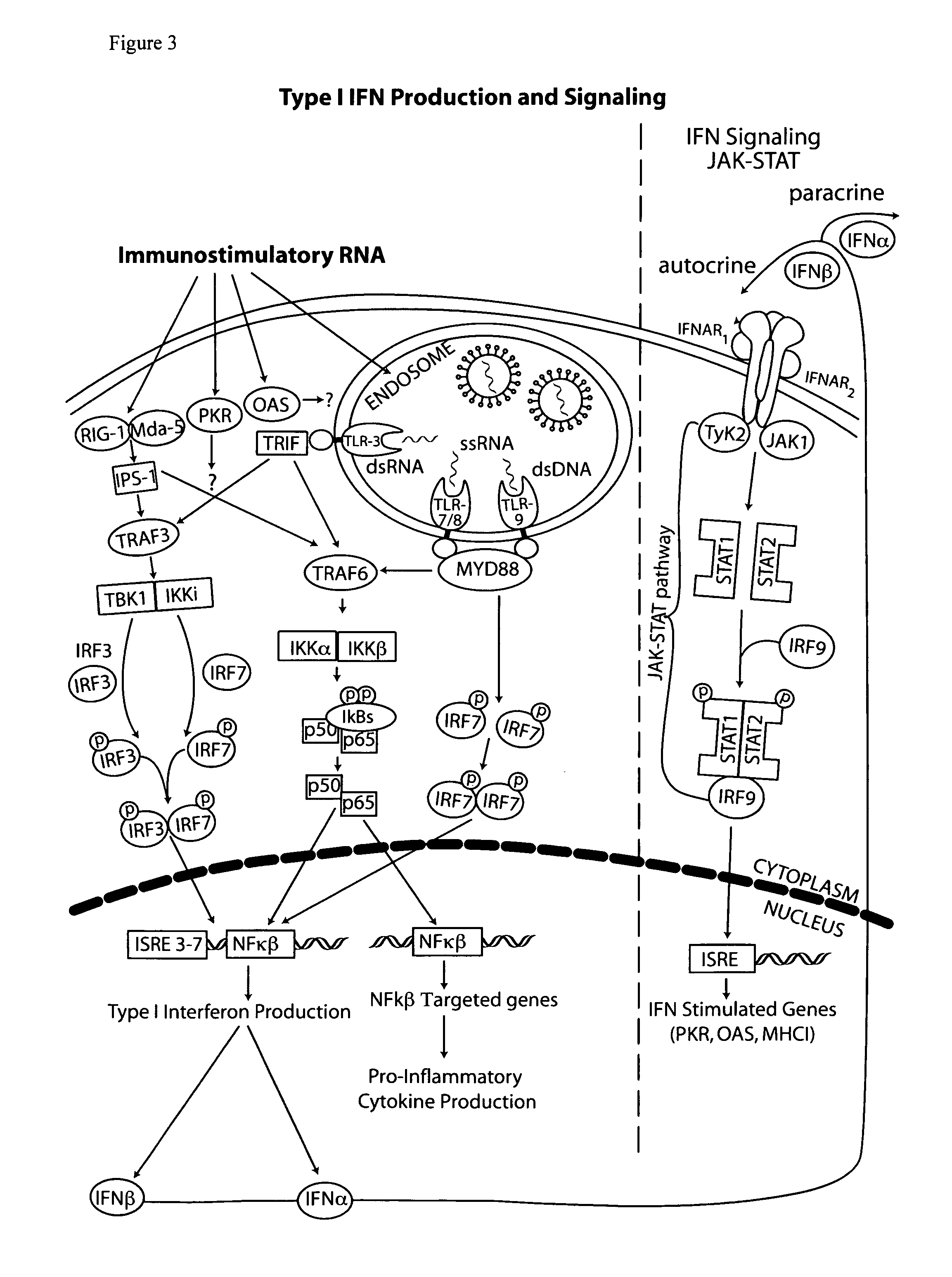
The type 1 interferons are a class of molecules that include IFNα and IFNβ. After binding to the type 1 interferon receptor (IFNAR) on target cells, these cytokines can stimulate the transcription of a set of genes, the type 1 interferon-inducible genes.
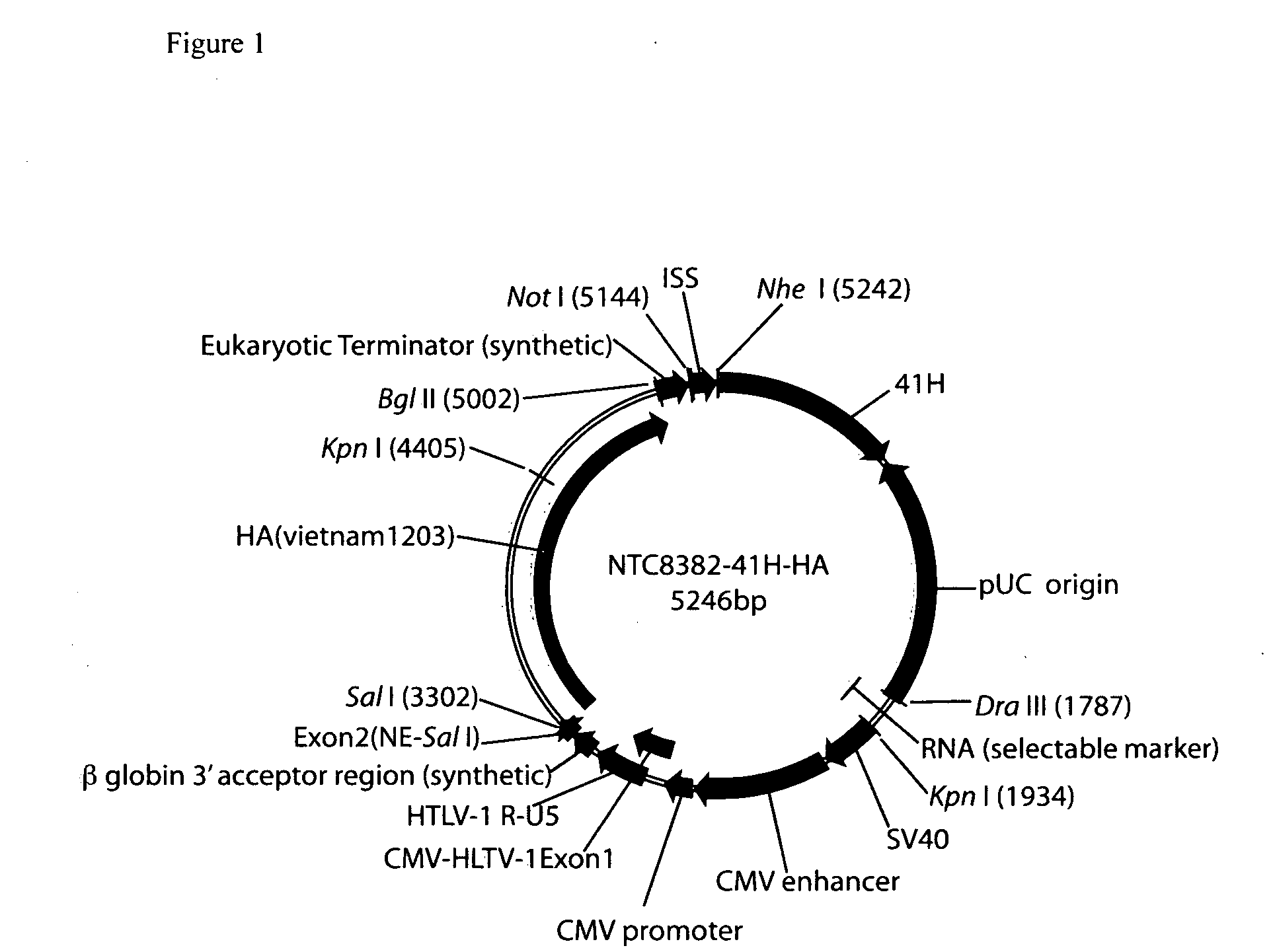
Vectors and method for genetic immunization
ActiveUS20100303859A1Small sizeImprove complianceSsRNA viruses negative-senseActivity regulationAntibiotic freeType 1 interferon
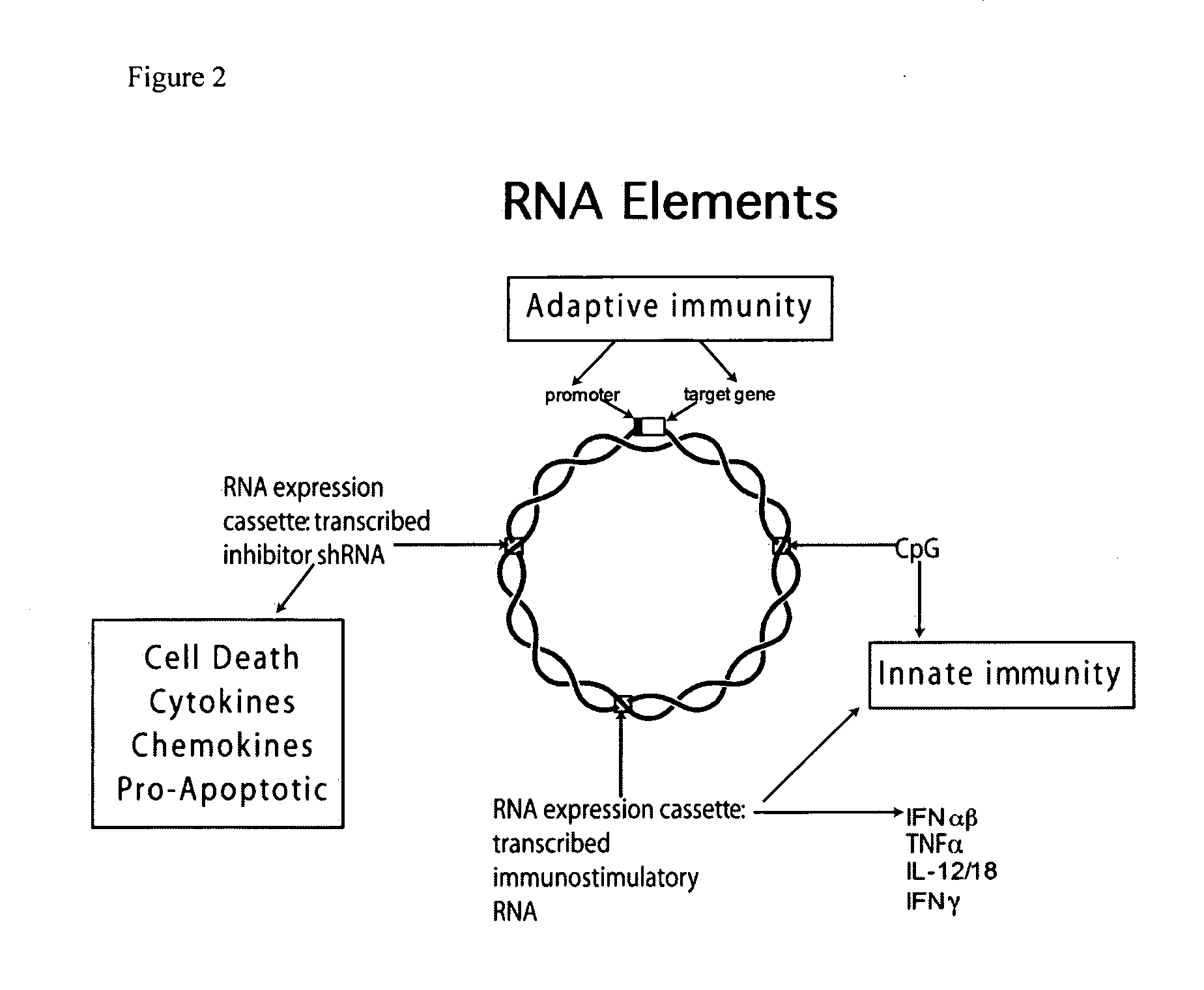
Improved DNA vaccine plasmids are disclosed that contain novel immunostimulatory RNA compositions. The improved plasmids eliminate all extraneous sequences, incorporate a novel antibiotic free short RNA based selectable marker, increase eukaryotic expression using a novel chimeric promoter, improve yield and stability during bacterial production, and improve immunostimulation. These vectors are utilized in immunization to elicit improved immune responses or therapy to induce type 1 interferon production.
Owner:ALDEVRON LLC
Type I Interferon Antagonists
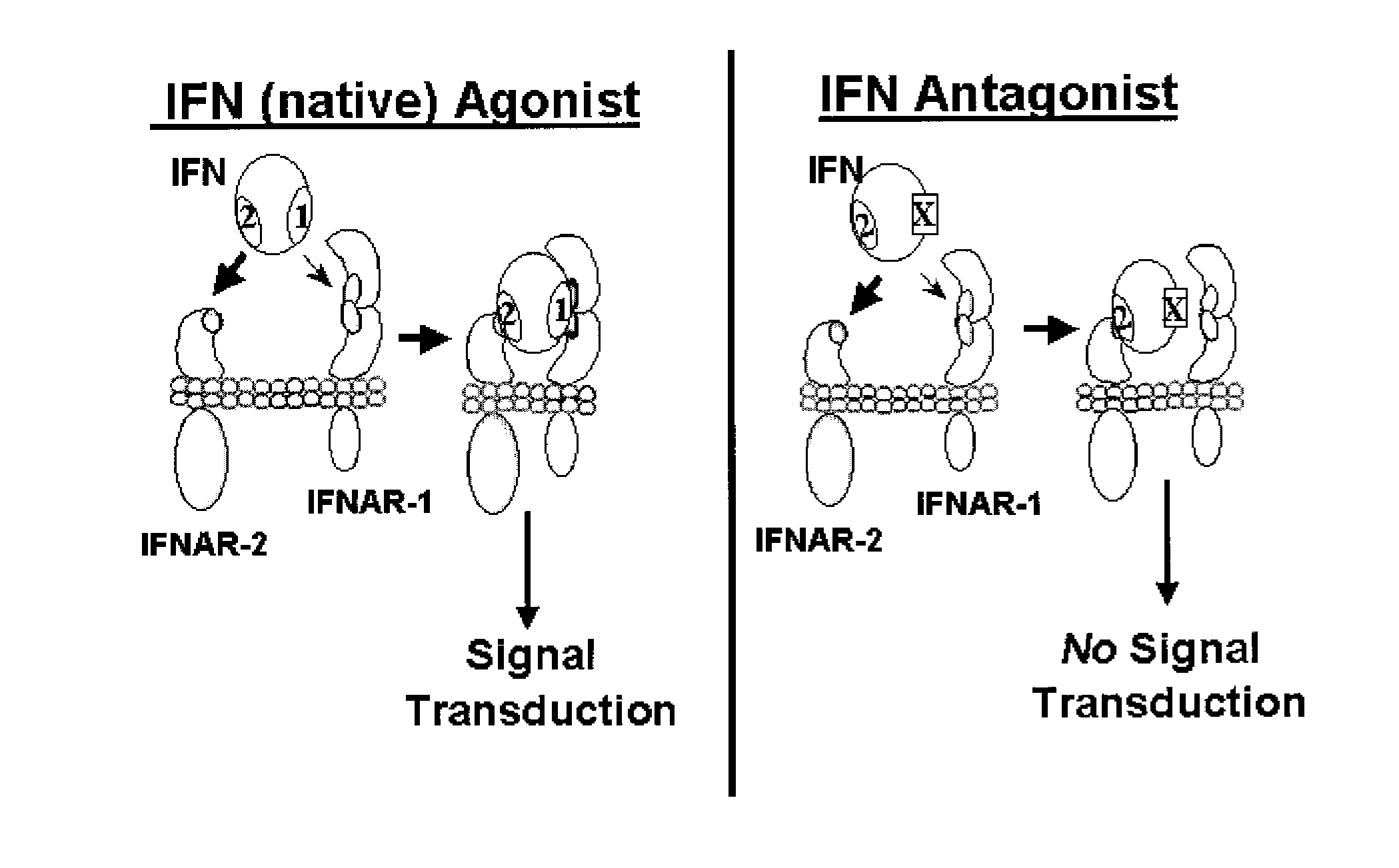
InactiveUS20110224407A1Binding affinityLow affinityDepsipeptidesPeptide preparation methodsInterferon receptorCancer research
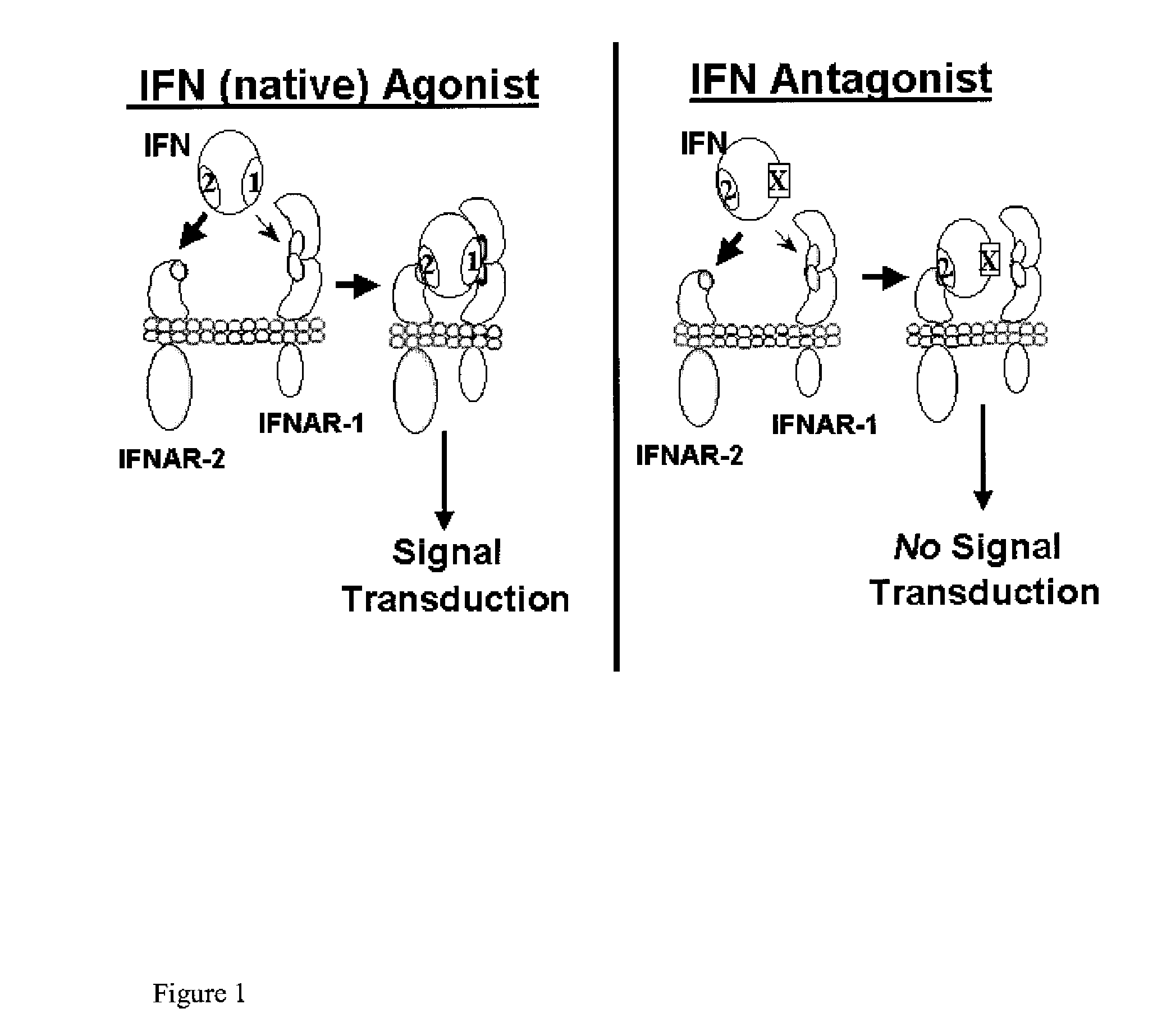
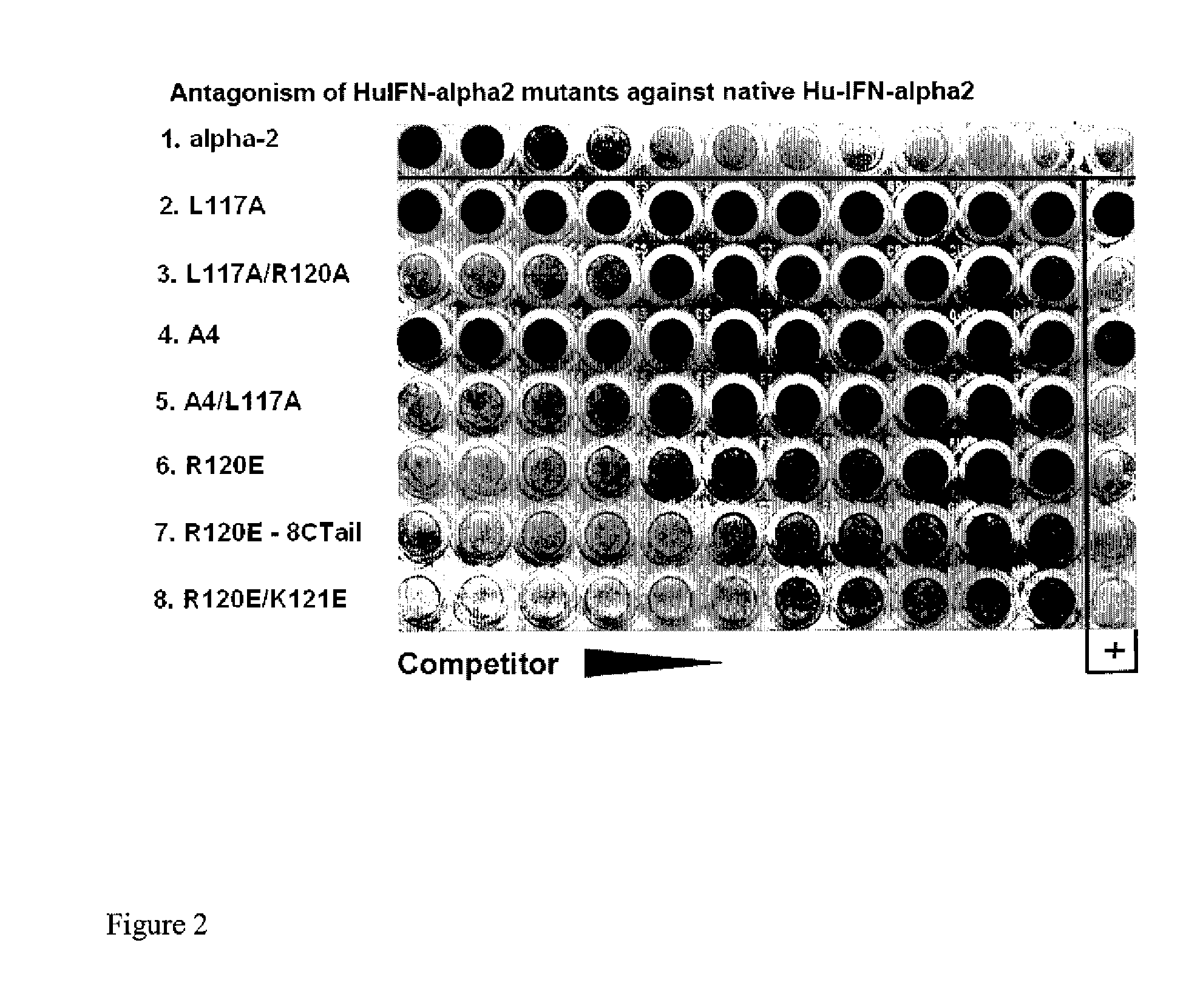
Disclosed in certain embodiments is a method of preparing a Type 1 interferon antagonist comprising modifying a Type 1 interferon at the site of interaction with the interferon receptor subunit IFNAR-1 such that the binding affinity of the interferon to the IFNAR-1 subunit is reduced as compared to the native interferon, and corresponding compositions and methods of treatment thereof.
Owner:YEDA RES & DEV CO LTD +1
Antagonist of th-1 immunerresponse inducing cytokine for the treatment of autoimmune diseases
Oromucosal administration of antagonists of cytokines associated with stimulation or enhancement of T helper 1 cell responses, preferably for example a Type 1-interferon antibody, is disclosed for inhibition of prevention of autoimmune diseases.
Owner:PHARM PACIFIC PTY LTD
Adjuvant compositions
InactiveUS20100003280A1Safe and effectiveImproving immunogenicityAntibacterial agentsOrganic active ingredientsAntigen deliveryNucleic acid sequencing
Adjuvant compositions comprising type 1 interferon inducers, such as double-stranded RNA, in combination with antigen delivery systems and / or immunostimulatory molecules, such as immunostimulatory nucleic acid sequences, for enhancing the immune response of a coadministered antigen, are described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions and methods for the therapy of Inflammatory Bowel Disease
InactiveUS20050152901A1Peptide/protein ingredientsAntibody mimetics/scaffoldsUlcerative colitisInterferon receptor
Compositions and methods for the therapy of Inflammatory Bowel Disease (IBD), including Celiac Disease, Crohn's Disease, and Ulcerative Colitis, are disclosed. Illustrative compositions comprise one or more anti-type 1 interferon antagonists, such as anti-type 1 interferon receptor antibody antagonists and fragments thereof, as well as polypeptides and small molecules that inhibit the interaction of type 1 interferon with its receptor (IFNAR).
Owner:ER SQUIBB & SONS INC
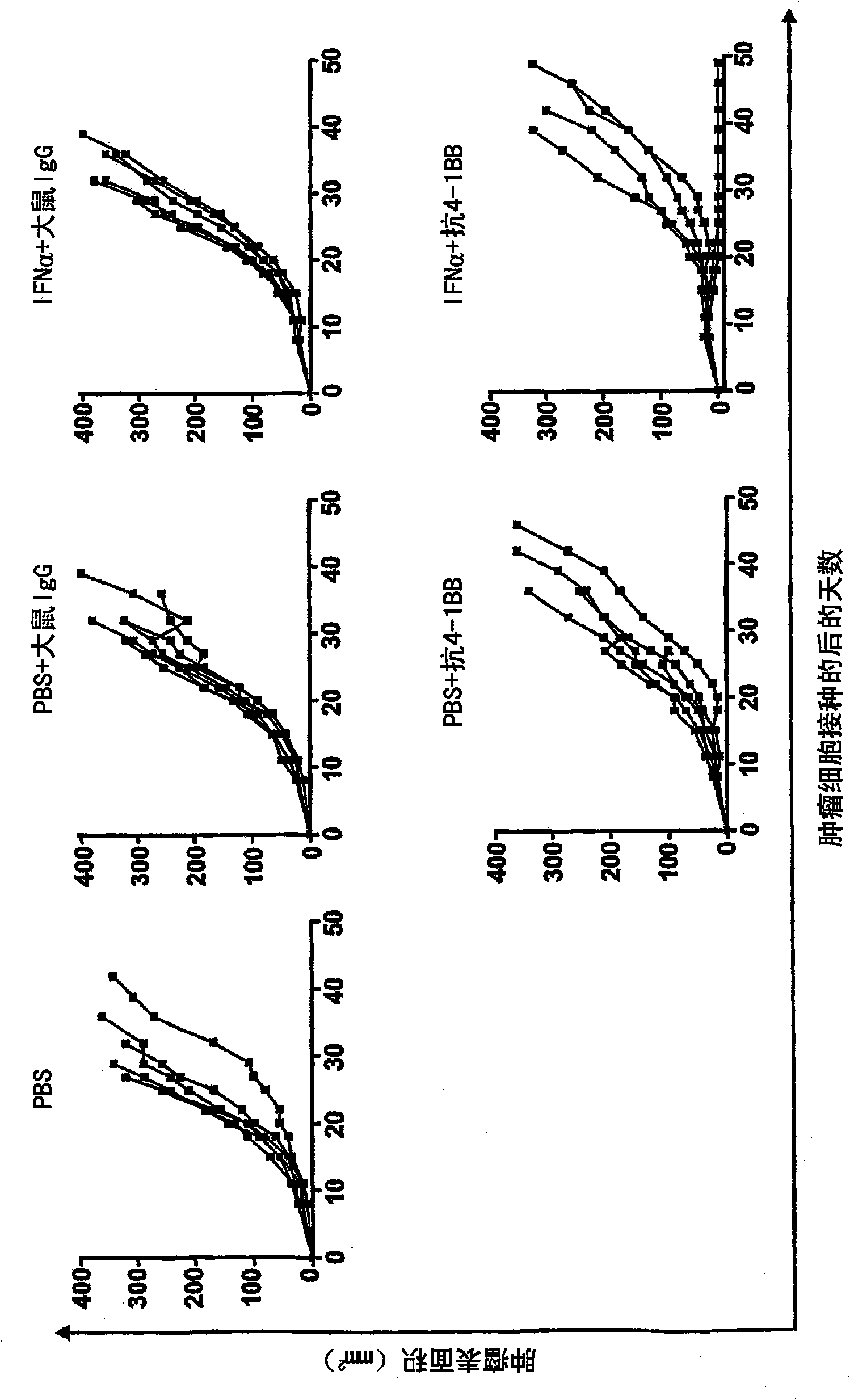
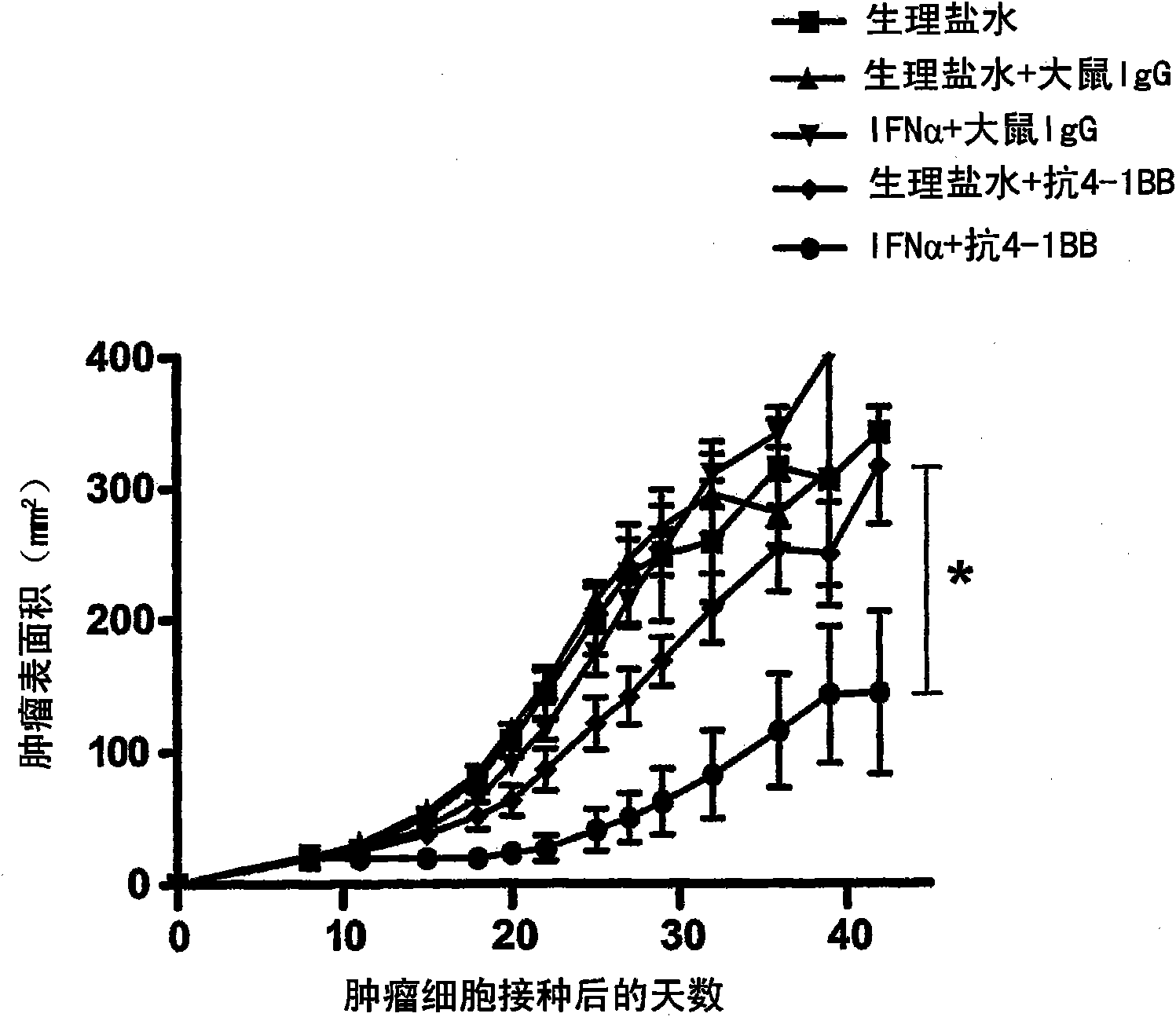
Pharmaceutical composition for cancer treatment
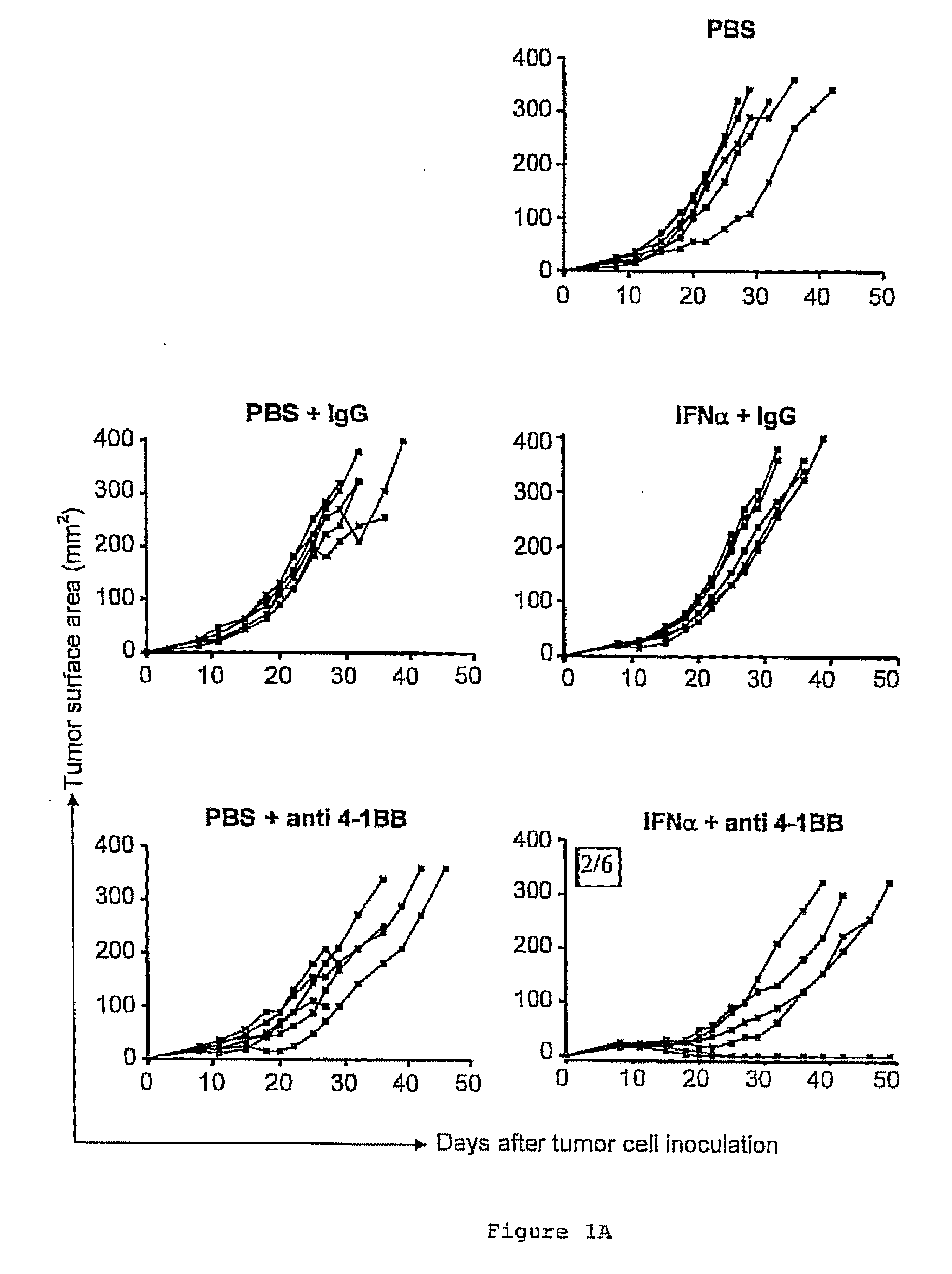
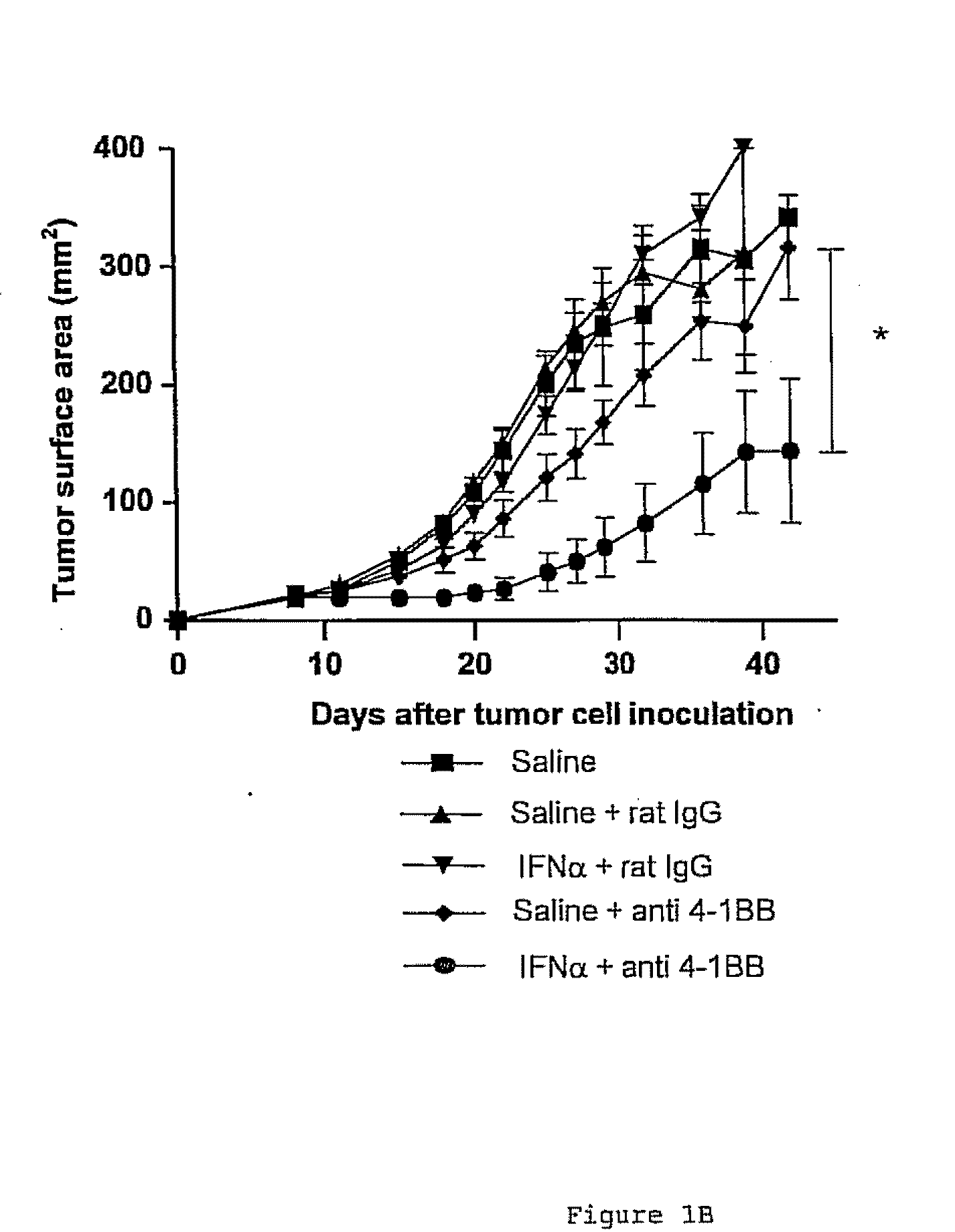
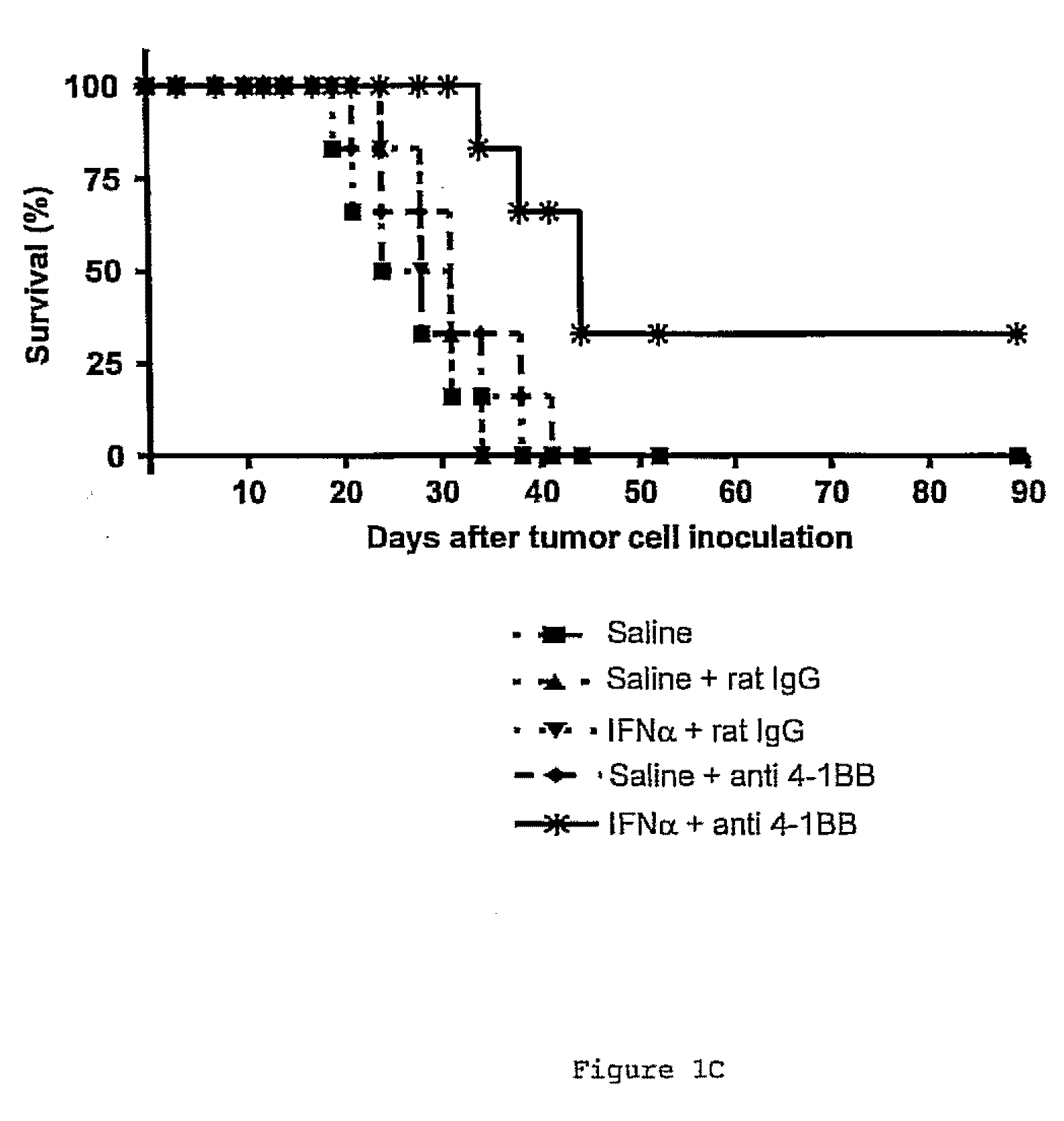
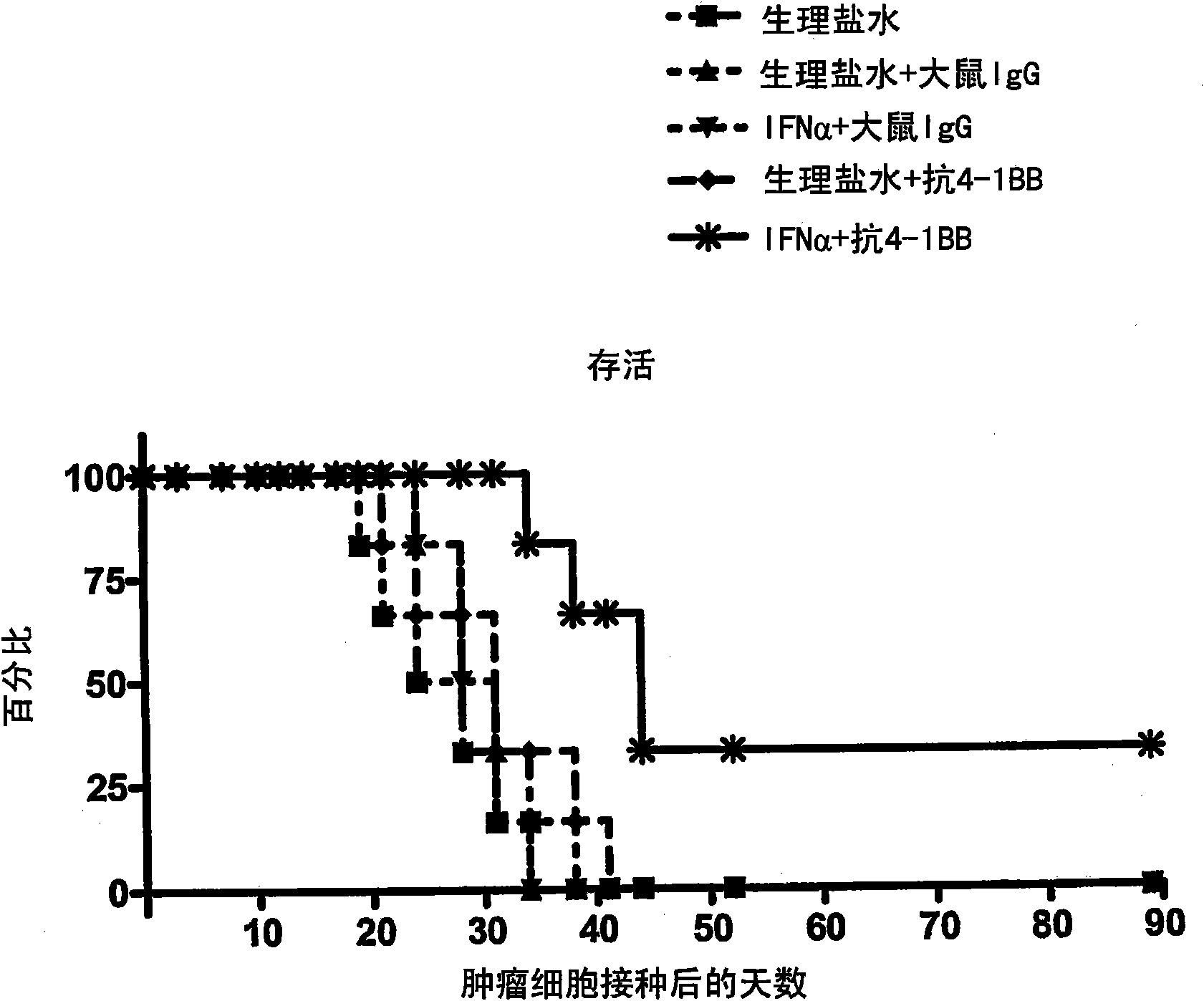
The invention relates to therapeutic compositions for the treatment of cancer and, more specifically, compositions containing an agonist ligand for receptor 4-1BB and a type-1 interferon, the simultaneous or sequential delivery of which results in a synergic antitumour effect in relation to the individual delivery of any of the components. The invention also relates to the therapeutic uses of the combinations of the invention for the treatment of cancer. The invention further relates to polynucleotides that code for compounds, vectors and cells containing same, as well as to the use thereof for the treatment of cancer.
Owner:PROYECTO DE BIOMEDICINA CIMA +1
Methods for the therapy of Inflammatory Bowel Disease using a type-1 interferon antagonist
InactiveUS7939076B2Peptide/protein ingredientsAntibody mimetics/scaffoldsCrohn's diseaseInflammatory Bowel Diseases
Compositions and methods for the therapy of Inflammatory Bowel Disease (IBD), including Celiac Disease, Crohn's Disease, and Ulcerative Colitis, are disclosed. Illustrative compositions comprise one or more anti-type 1 interferon antagonists, such as anti-type 1 interferon receptor antibody antagonists and fragments thereof, as well as polypeptides and small molecules that inhibit the interaction of type 1 interferon with its receptor (IFNAR).
Owner:ER SQUIBB & SONS INC
Compositions and methods for targeting type 1 interferon producing cells
ActiveUS20130084282A1In-vivo radioactive preparationsPeptide/protein ingredientsPlasmacytoid dendritic cellSystemic lupus erythematosus
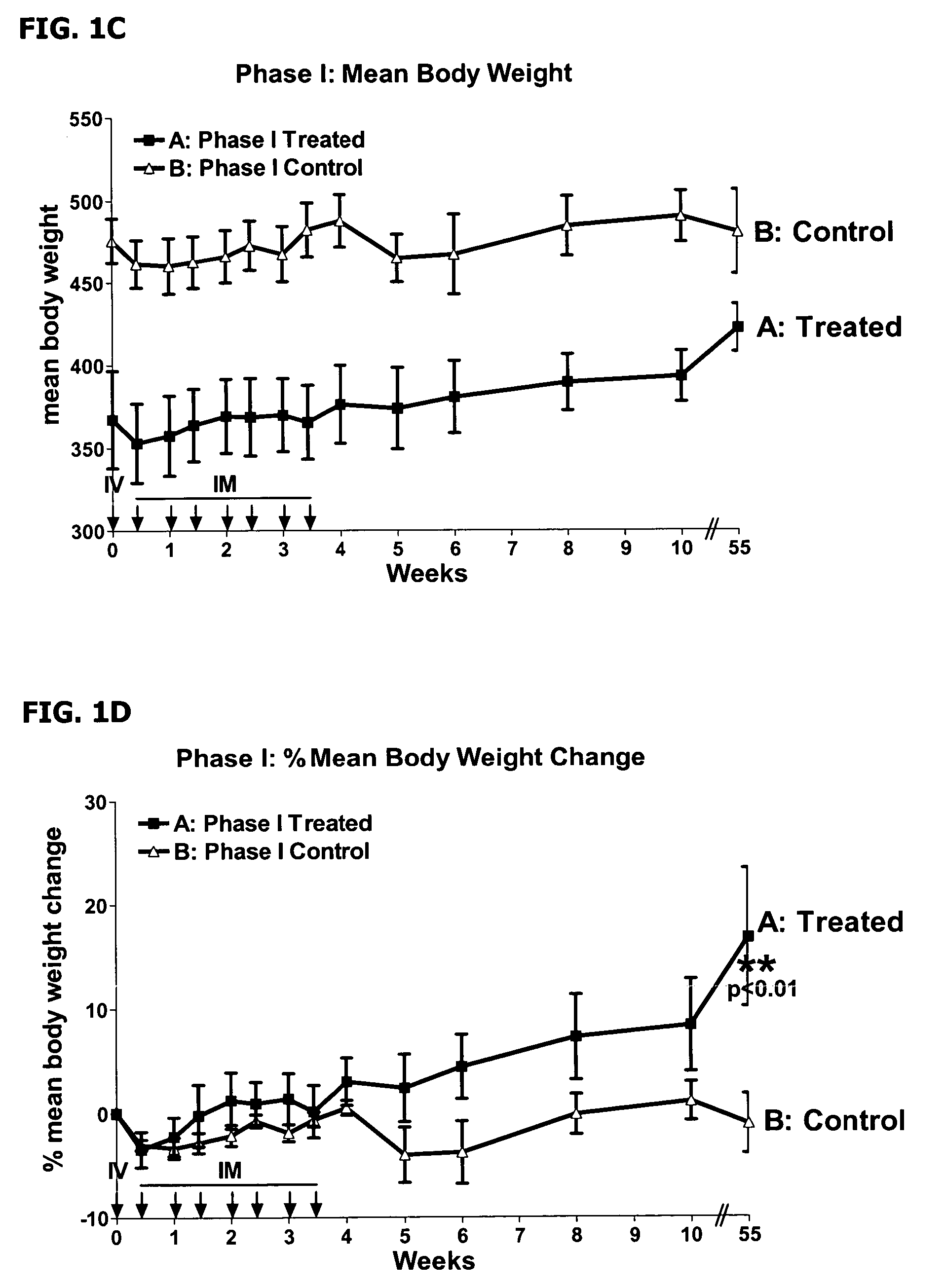
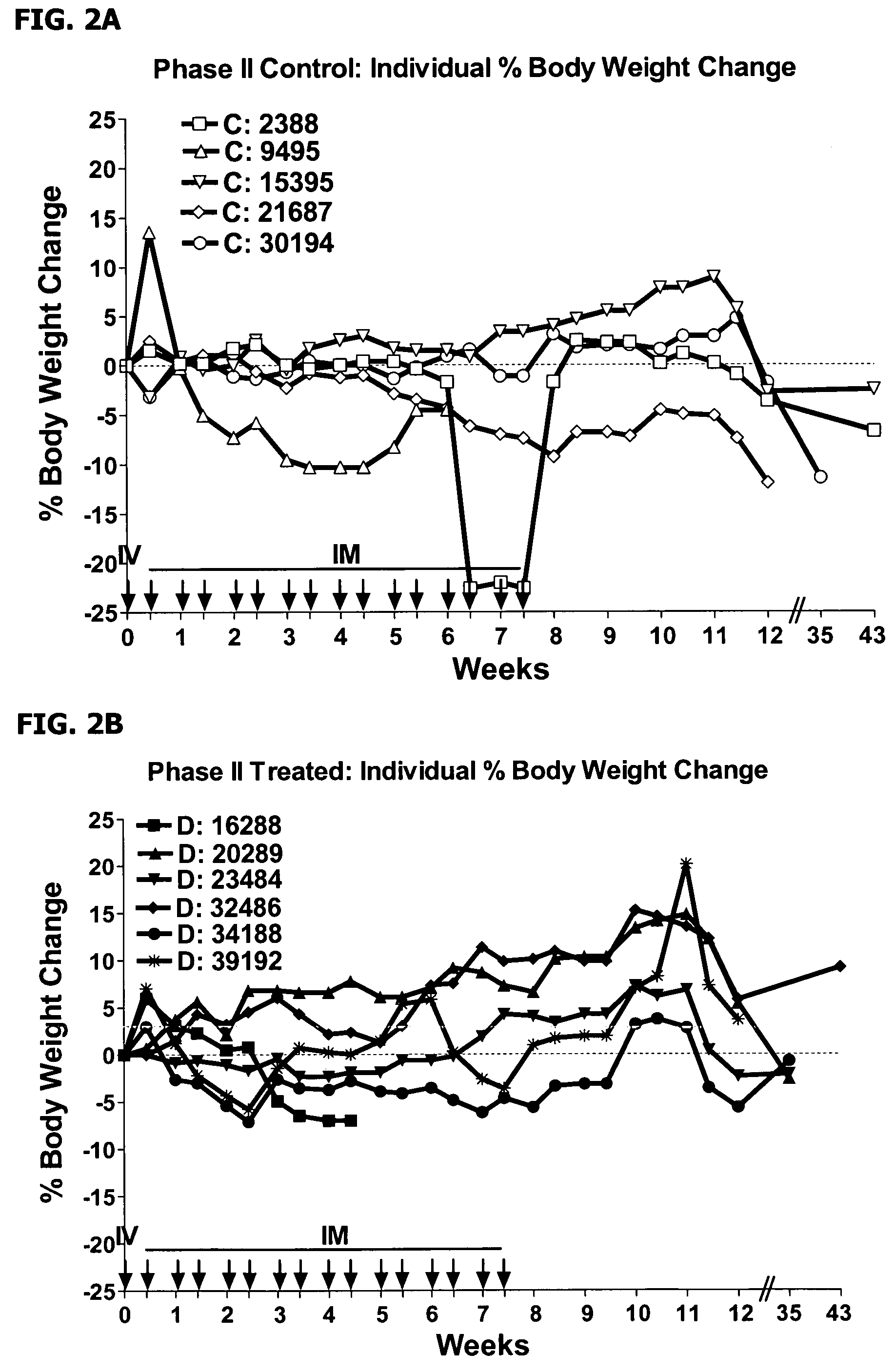
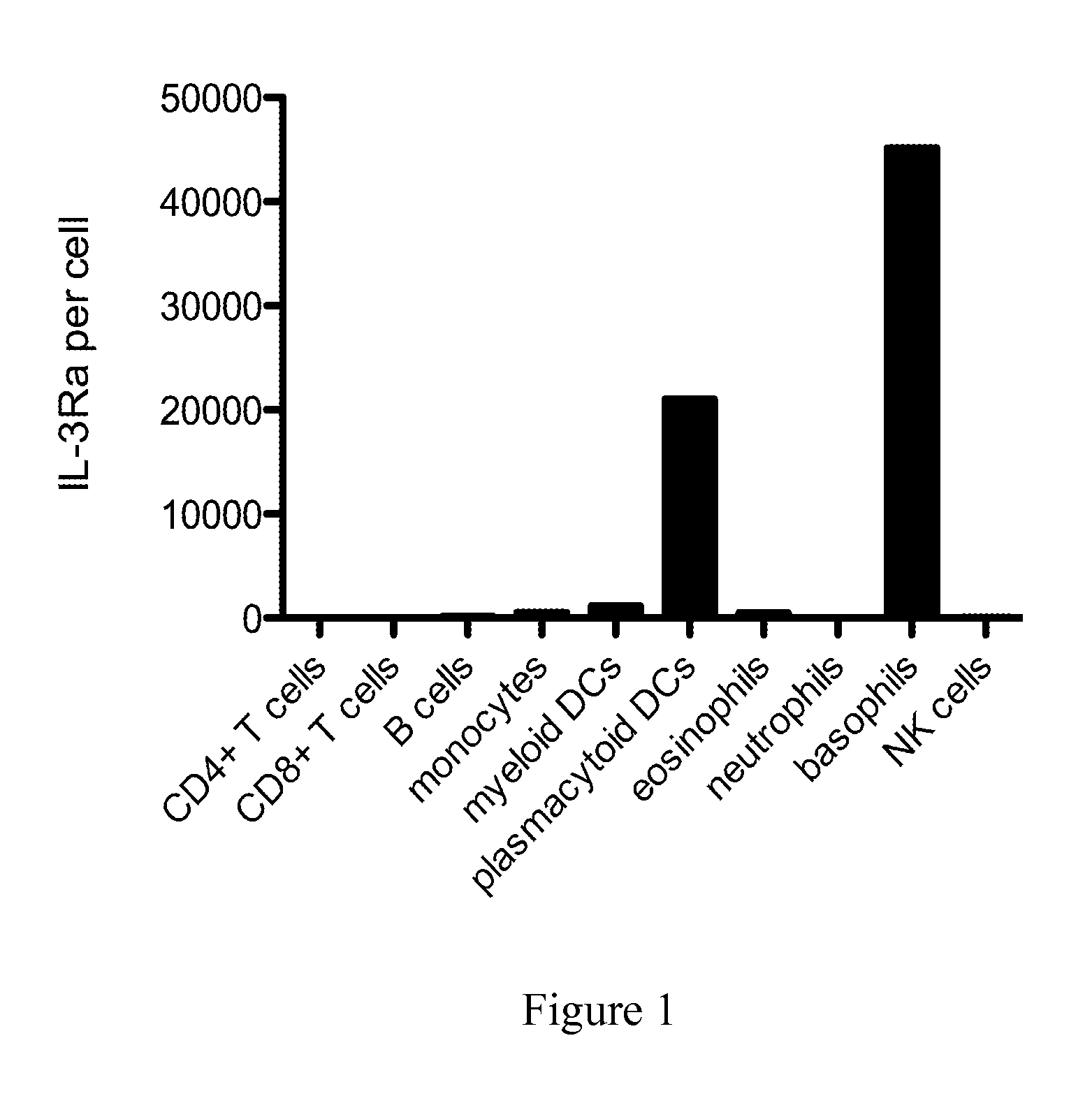
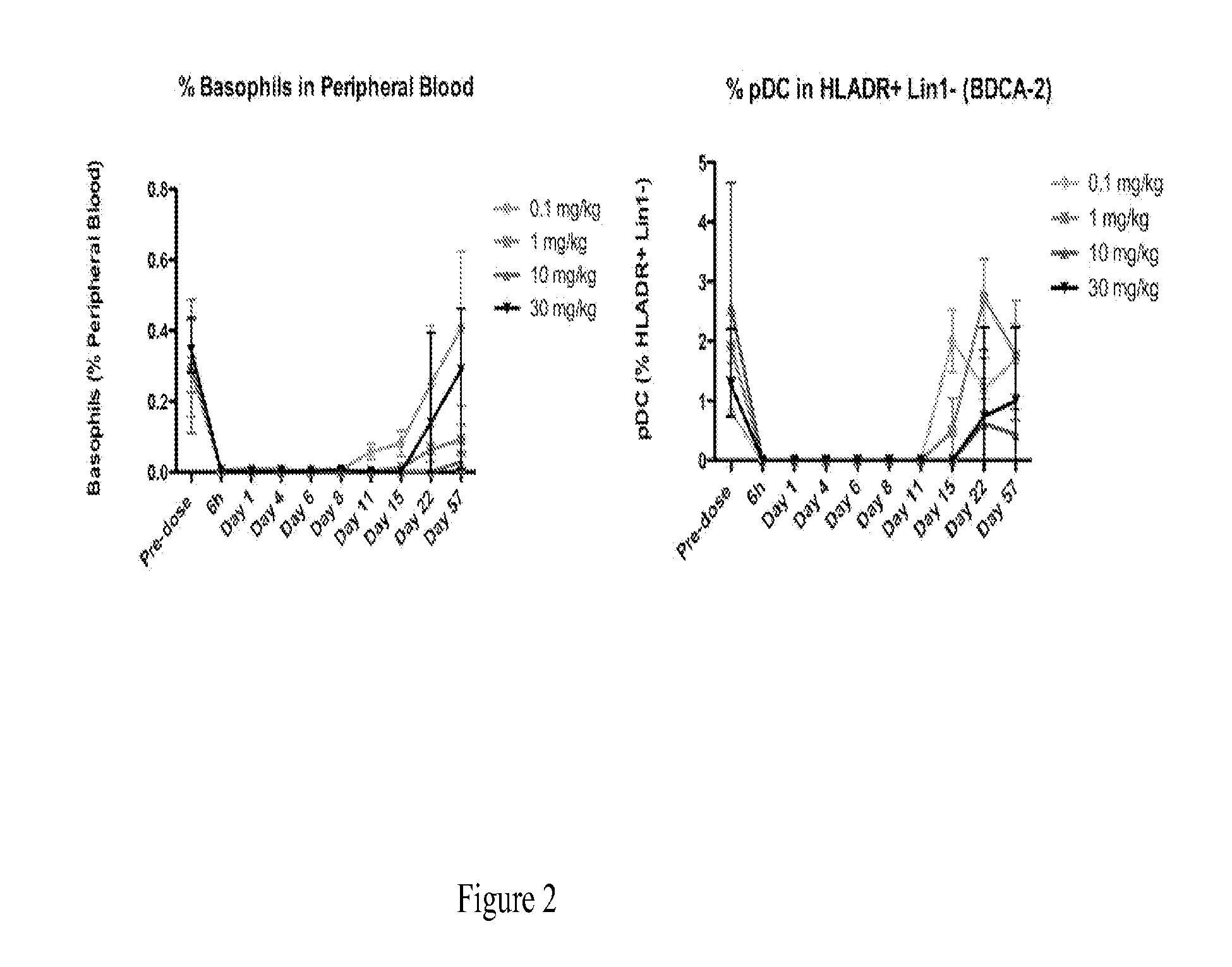
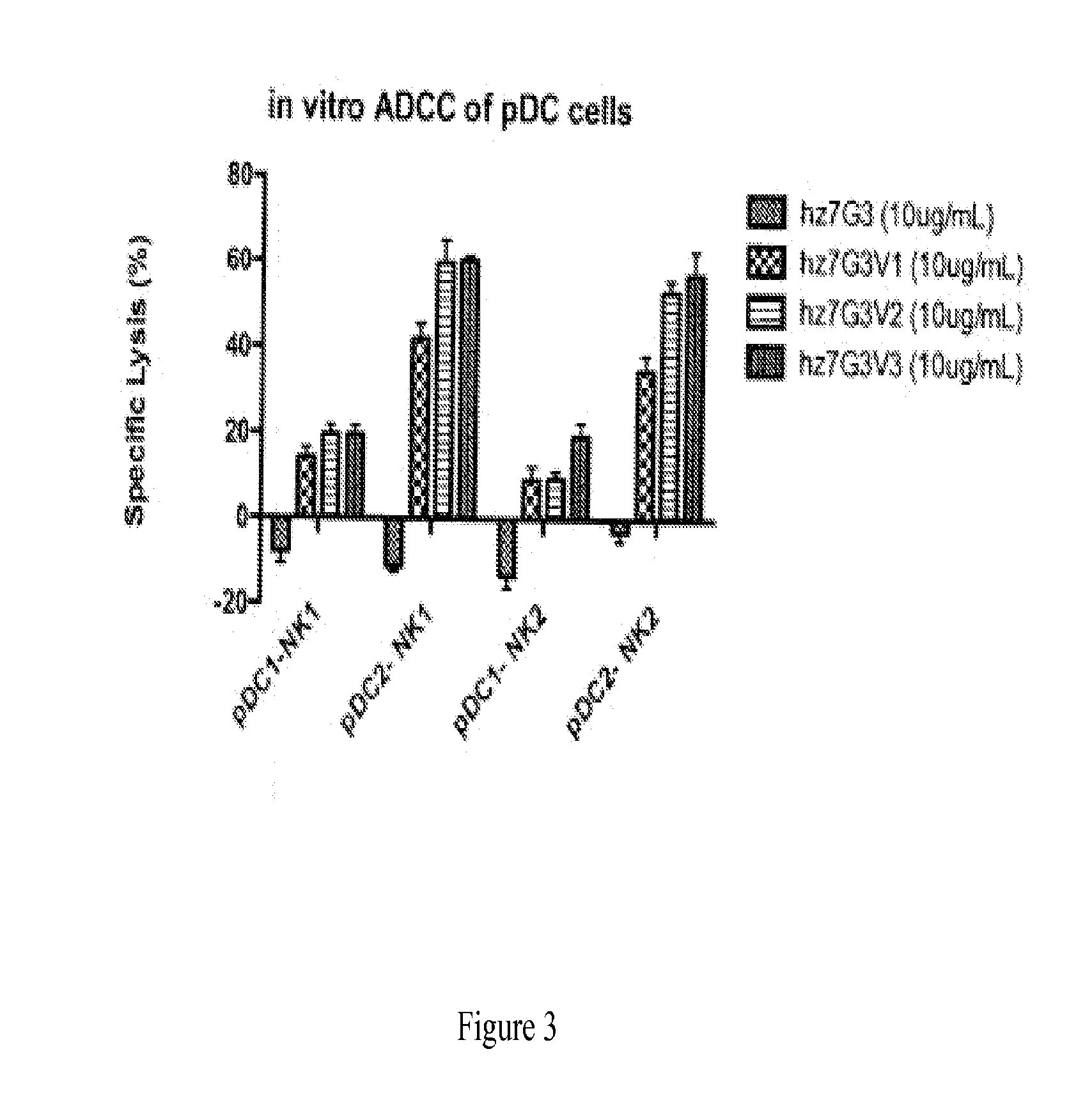
The present disclosure provides a method for treating lupus, Sjörgen's syndrome or scleroderma, the method comprising administering to the mammal an immunoglobulin which binds an interleukin 3 receptor α (IL-3Rα) chain and which depletes or at least partly eliminates plasmacytoid dendritic cells (p DCs) and basophils to which it binds.
Owner:CSL LTD
Interferon alpha responsive protein
The present invention relates to identification of a gene upregulated by interferon-α administration corresponding to the cDNA sequence set forth in SEQ ID NO: 1. Determination of expression products of this gene is proposed as having utility in predicting responsiveness to treatment with interferon-a and other interferons which act at the Type 1 interferon receptor. Therapeutic use of the protein encoded by the same gene is also envisaged.
Owner:PHARM PACIFIC PTY LTD
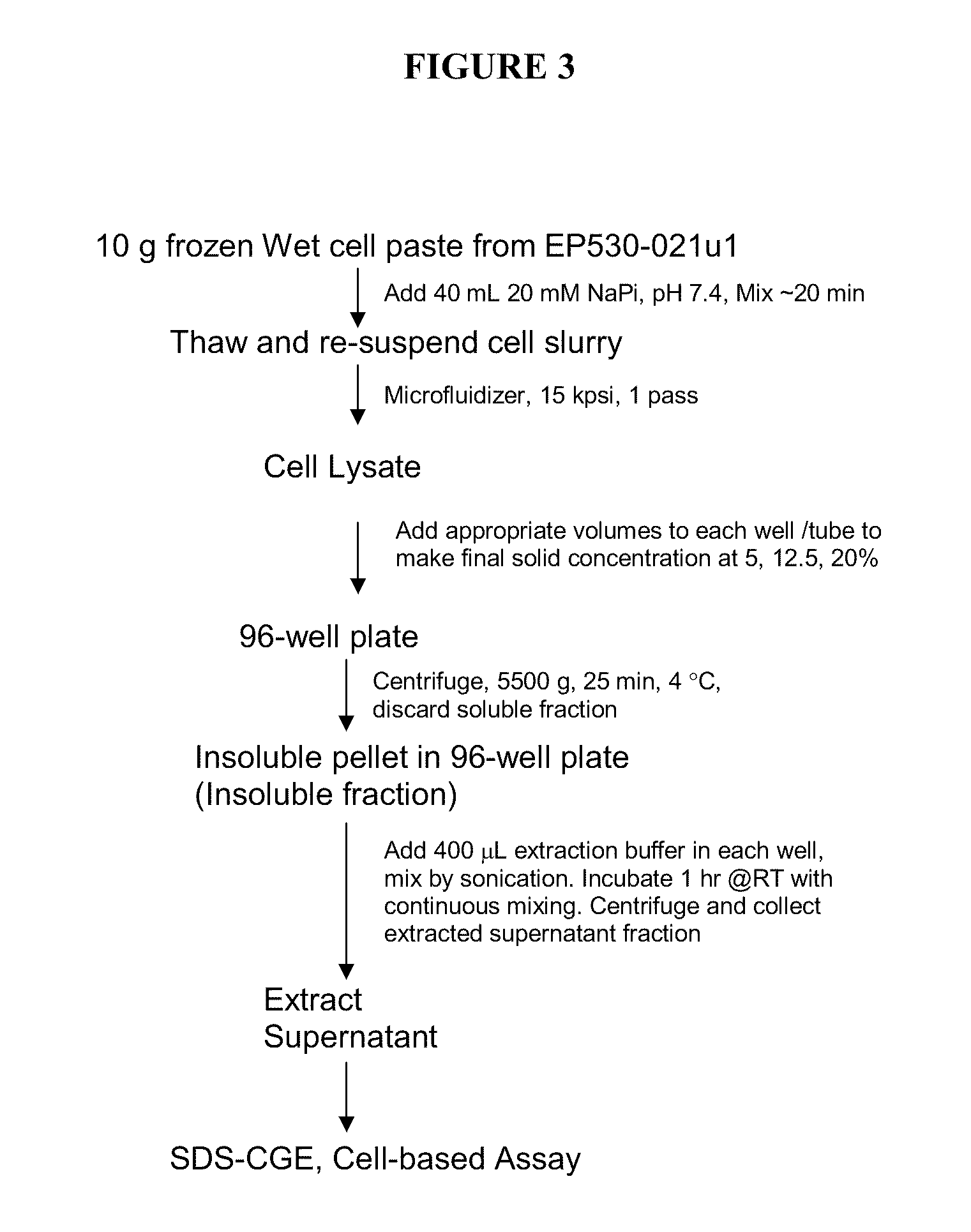
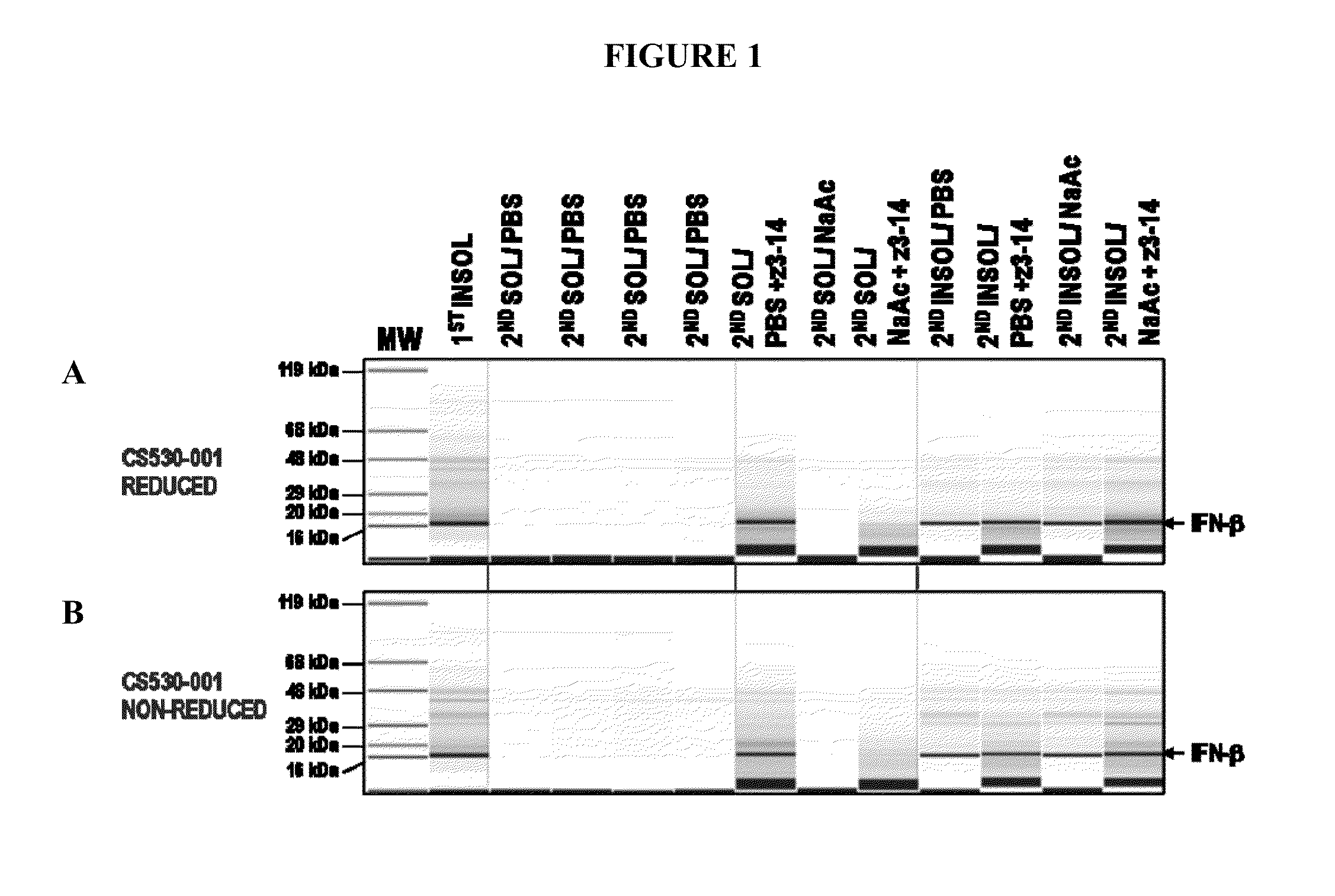
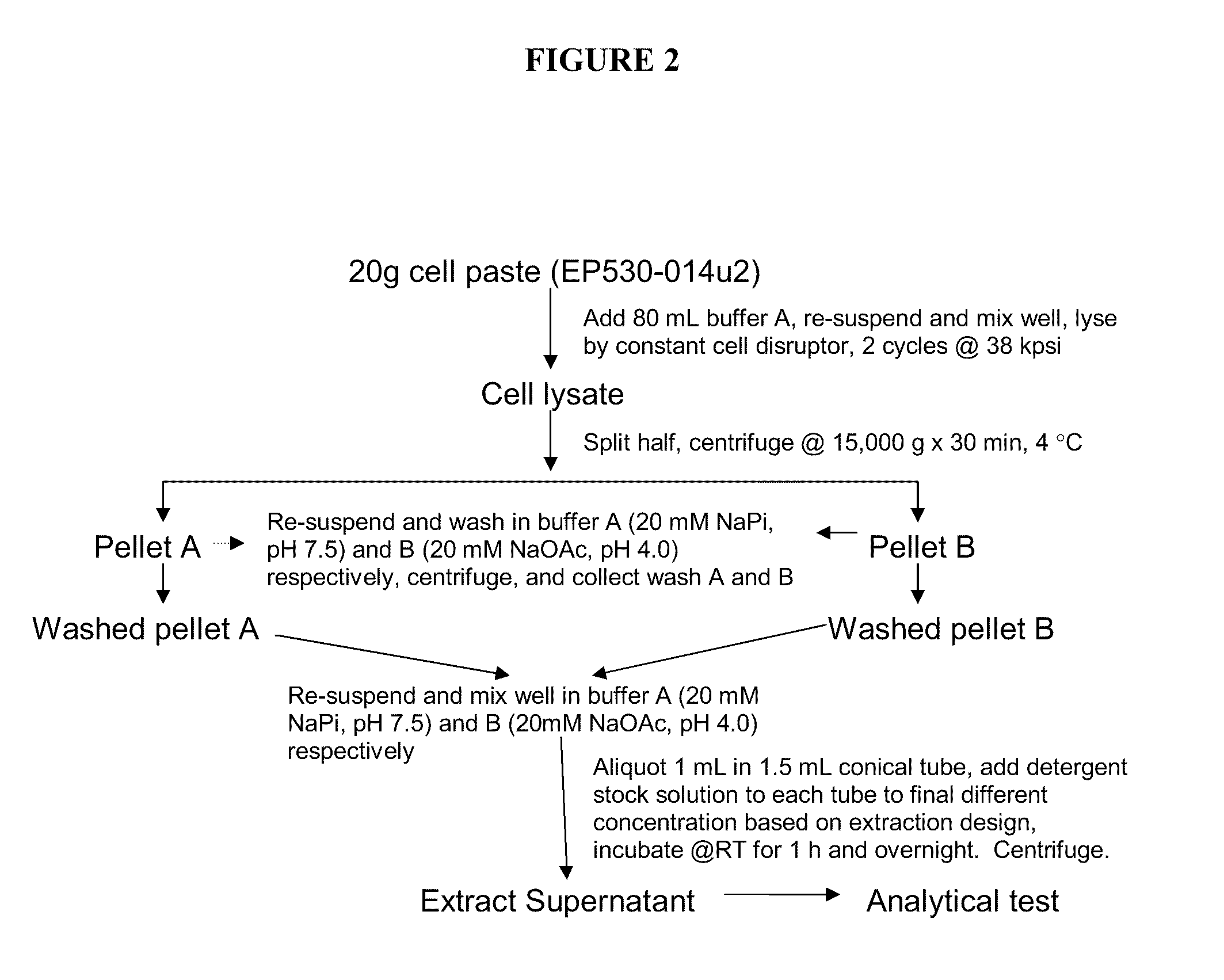
Method for producing soluble recombinant interferon protein without denaturing
ActiveUS20110217784A1Peptide/protein ingredientsPeptide preparation methodsType 1 interferonBacteria
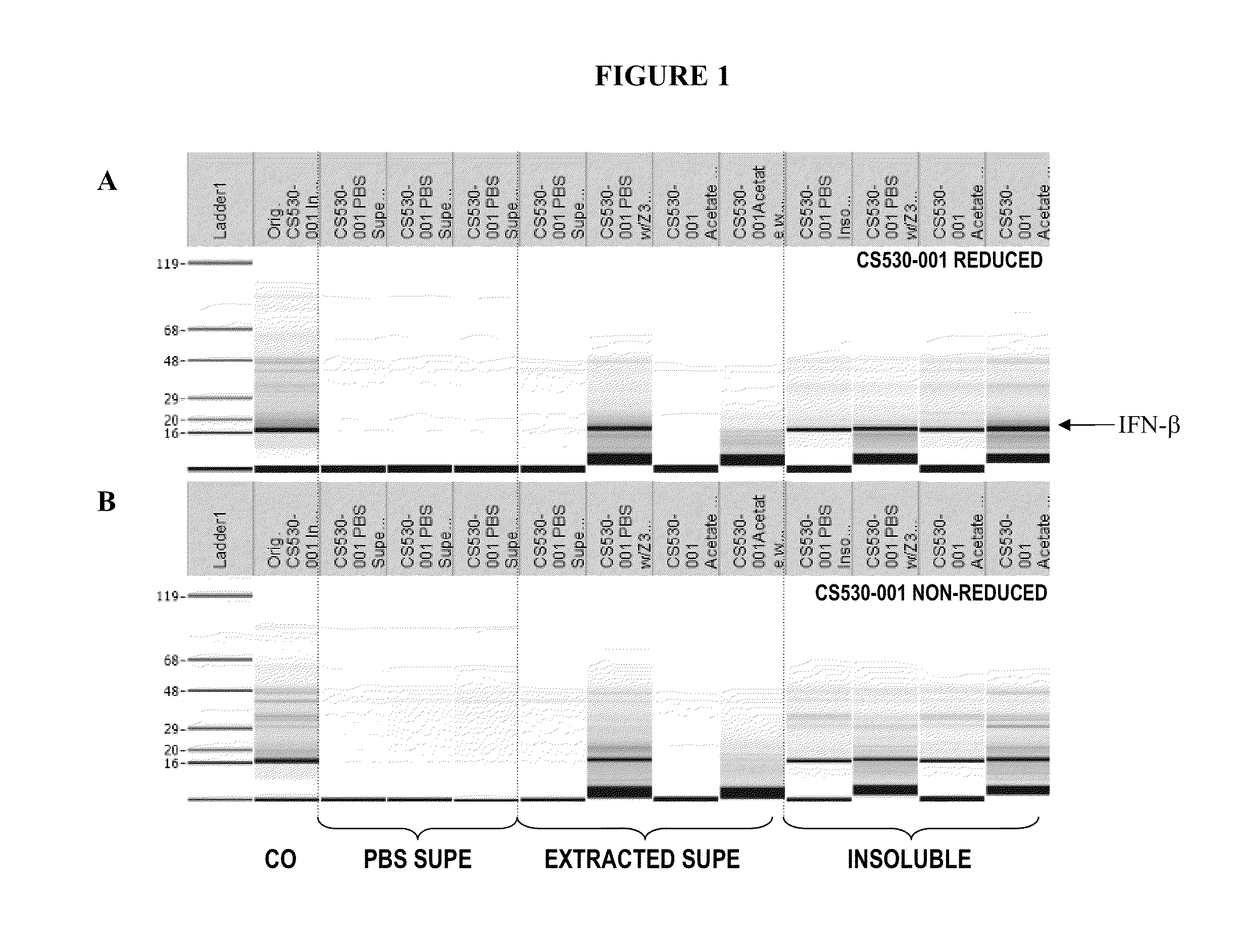
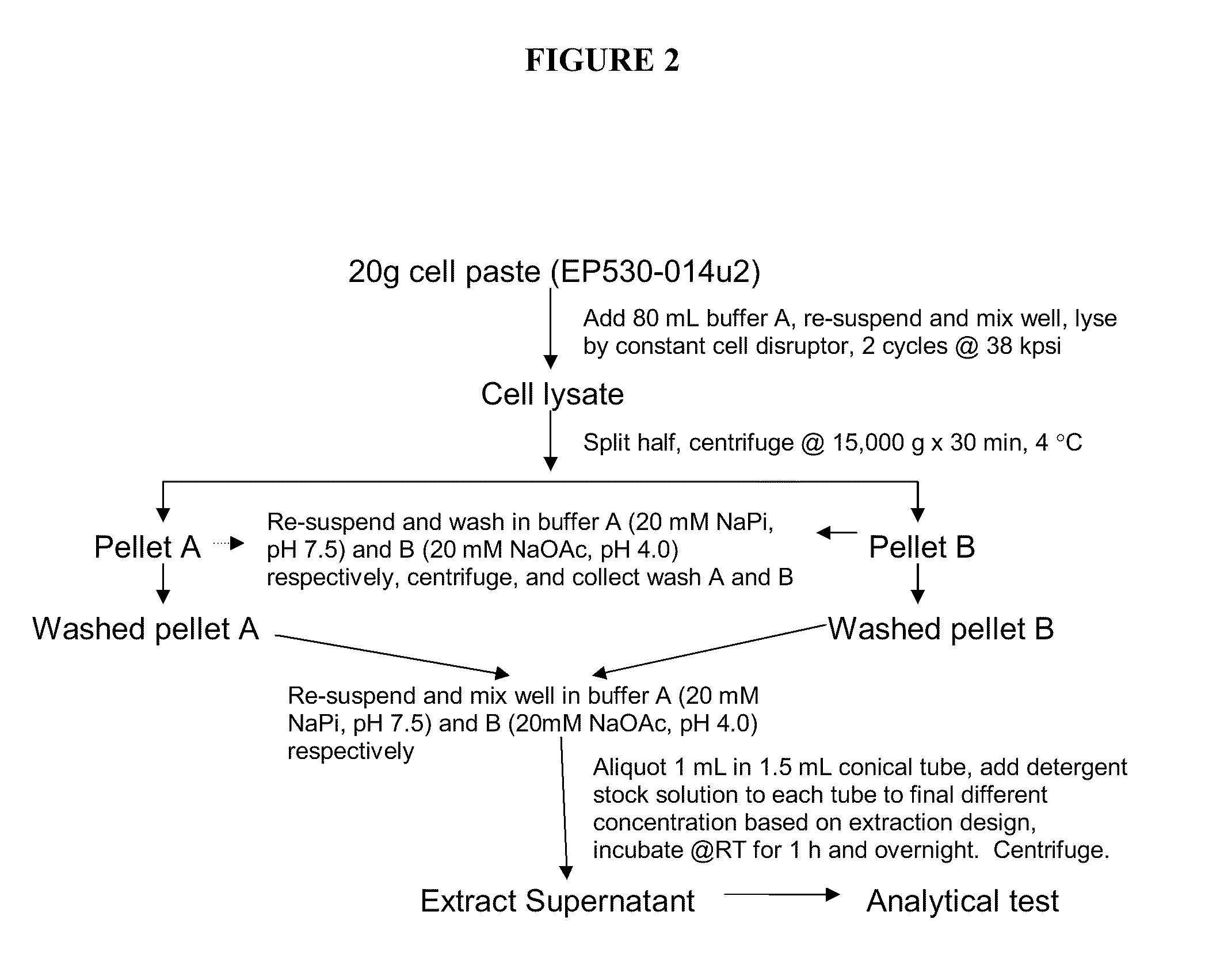
The present invention relates to the field of recombinant protein production in bacterial hosts. It further relates to extraction of soluble, active recombinant protein from an insoluble fraction without the use of denaturation and without the need for a refolding step. In particular, the present invention relates to a production process for obtaining high levels a soluble recombinant Type 1 interferon protein from a bacterial host.
Owner:PFENEX
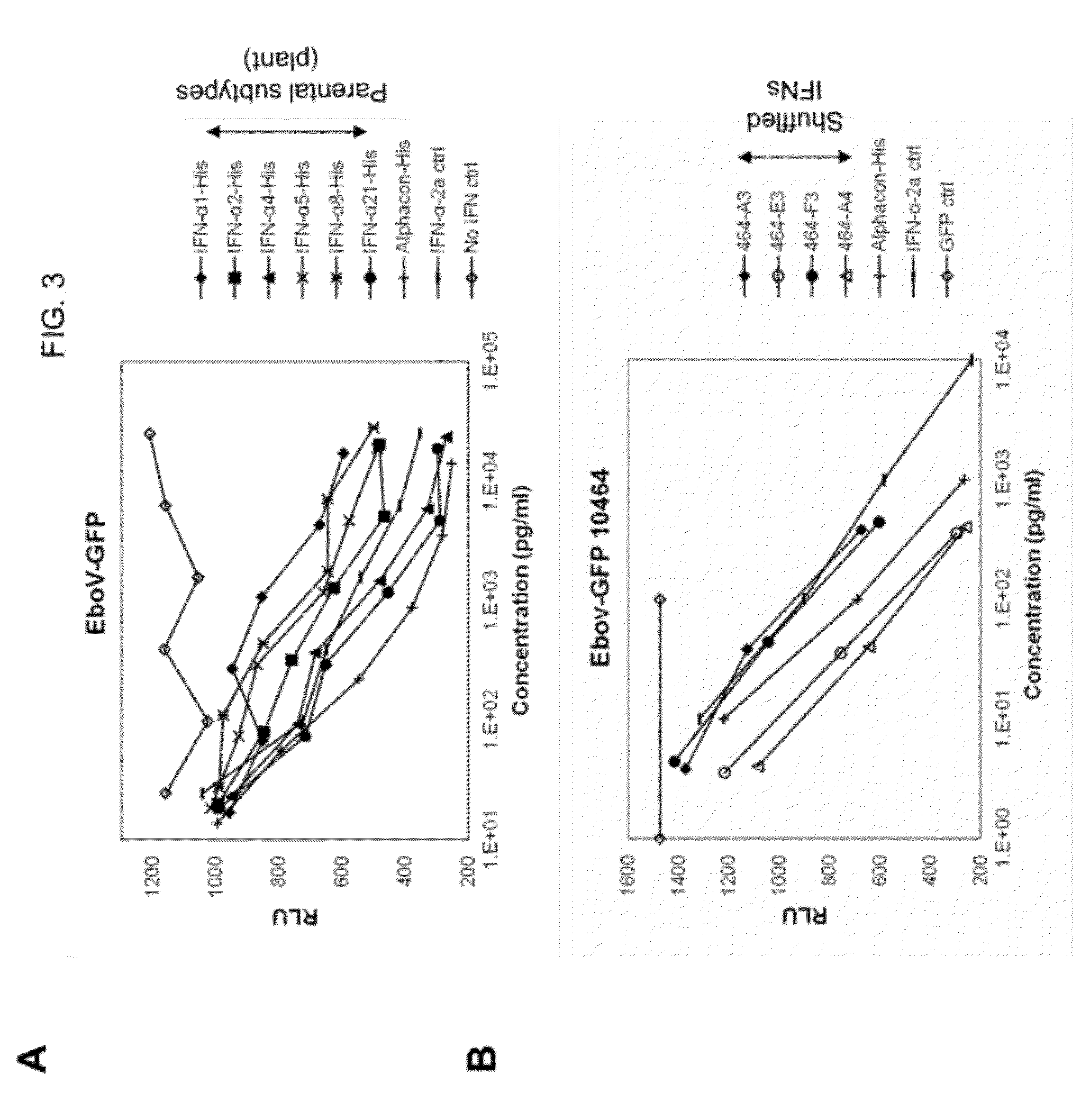
Selection and characterization of novel plant-derived recombinant human interferons with broad spectrum activity
InactiveUS20120302733A1Polypeptide with localisation/targeting motifPeptide preparation methodsHybrid typeHighly pathogenic
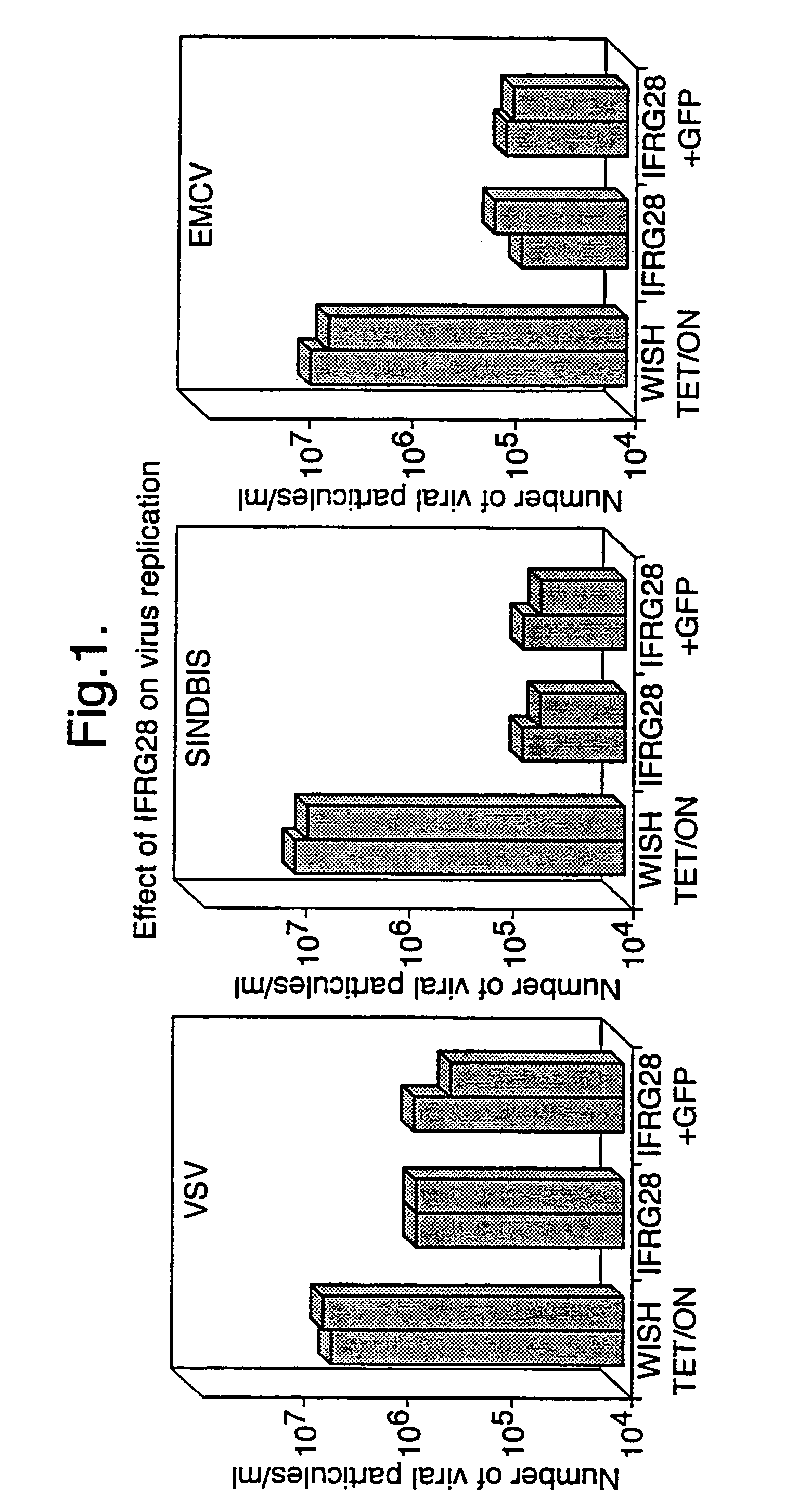
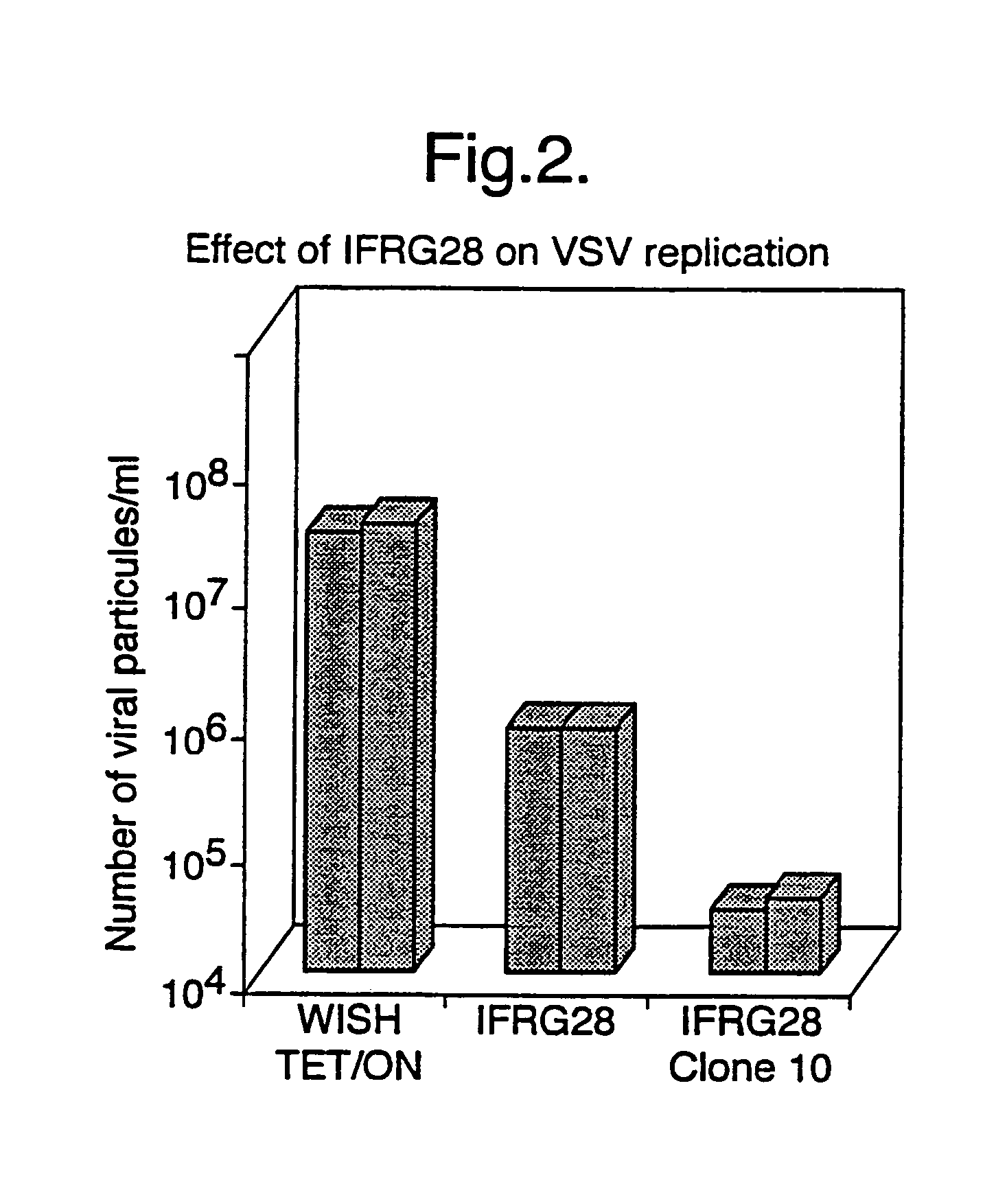
Methods to derive novel hybrid type 1 interferons that are broadly active against highly pathogenic viruses of biodefense significance are described. Libraries of hybrid interferon genes were produced using gene shuffling, the proteins were expressed, and screened for activity against viruses of interest. Sequences of several broadly active hybrid interferons are described.
Owner:PADGETT HAL S +2
Vectors and method for genetic immunization
ActiveUS9109012B2High expressionImprove integritySsRNA viruses negative-senseActivity regulationAntibiotic freeGenetics
Owner:ALDEVRON LLC
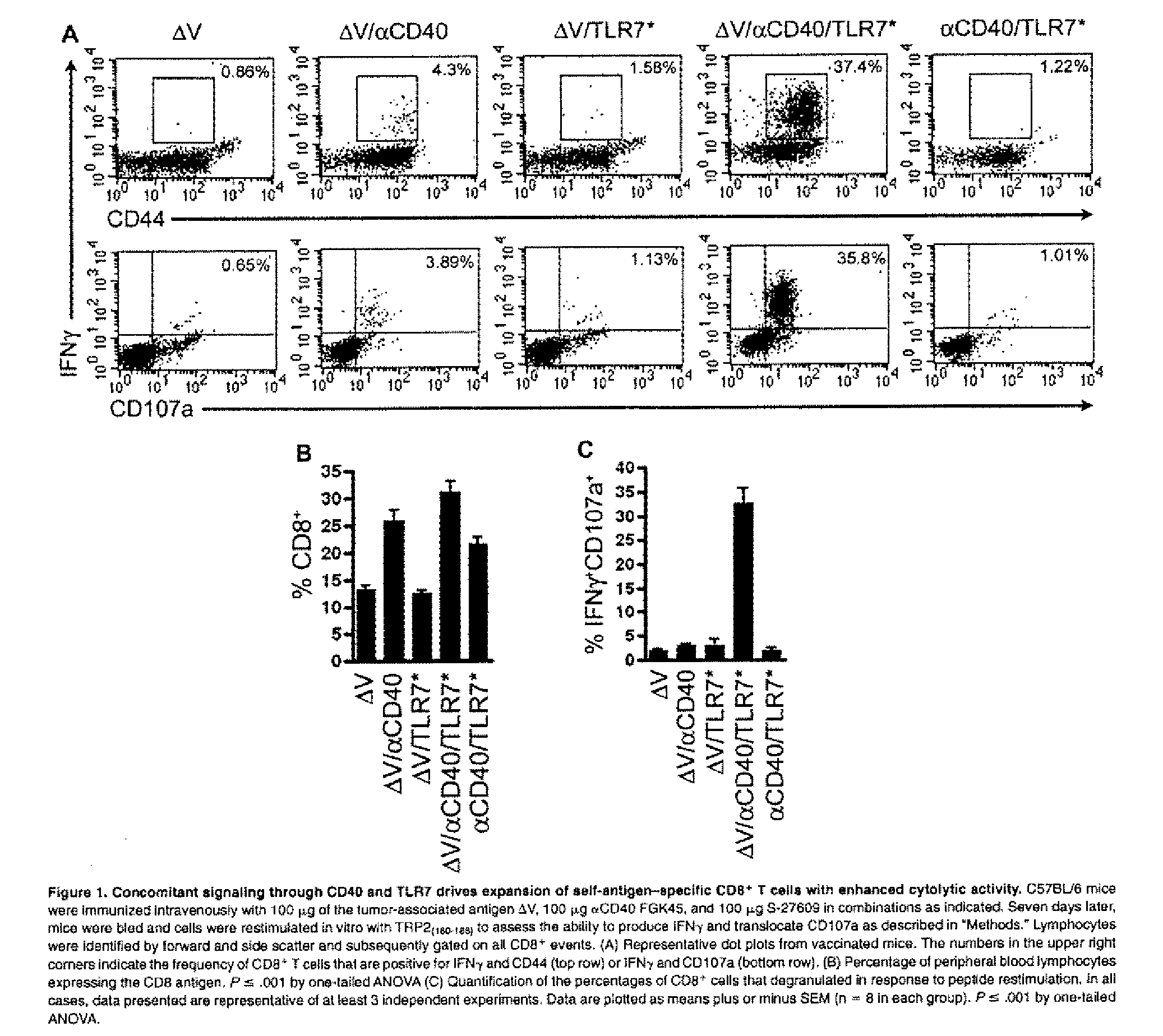
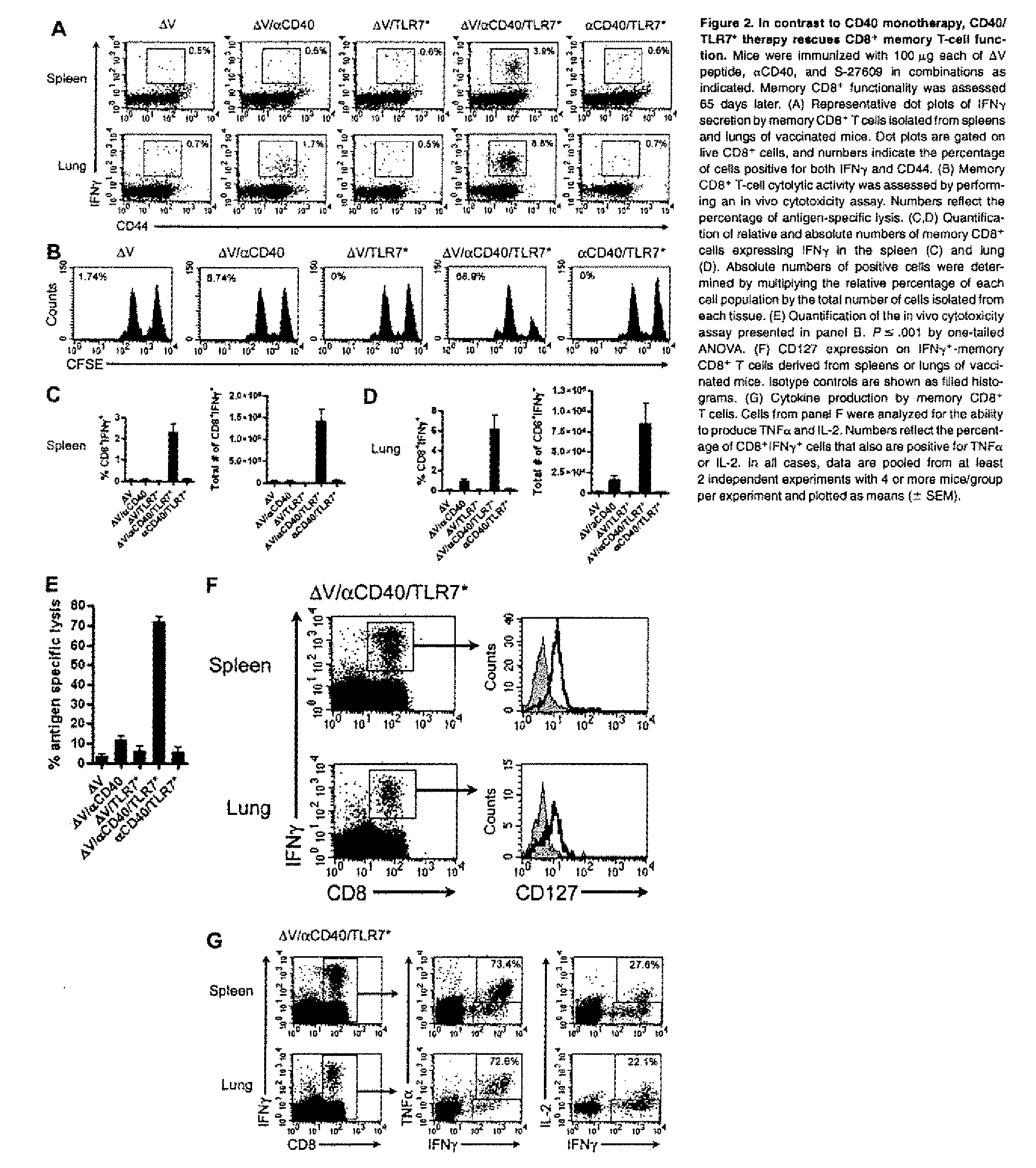
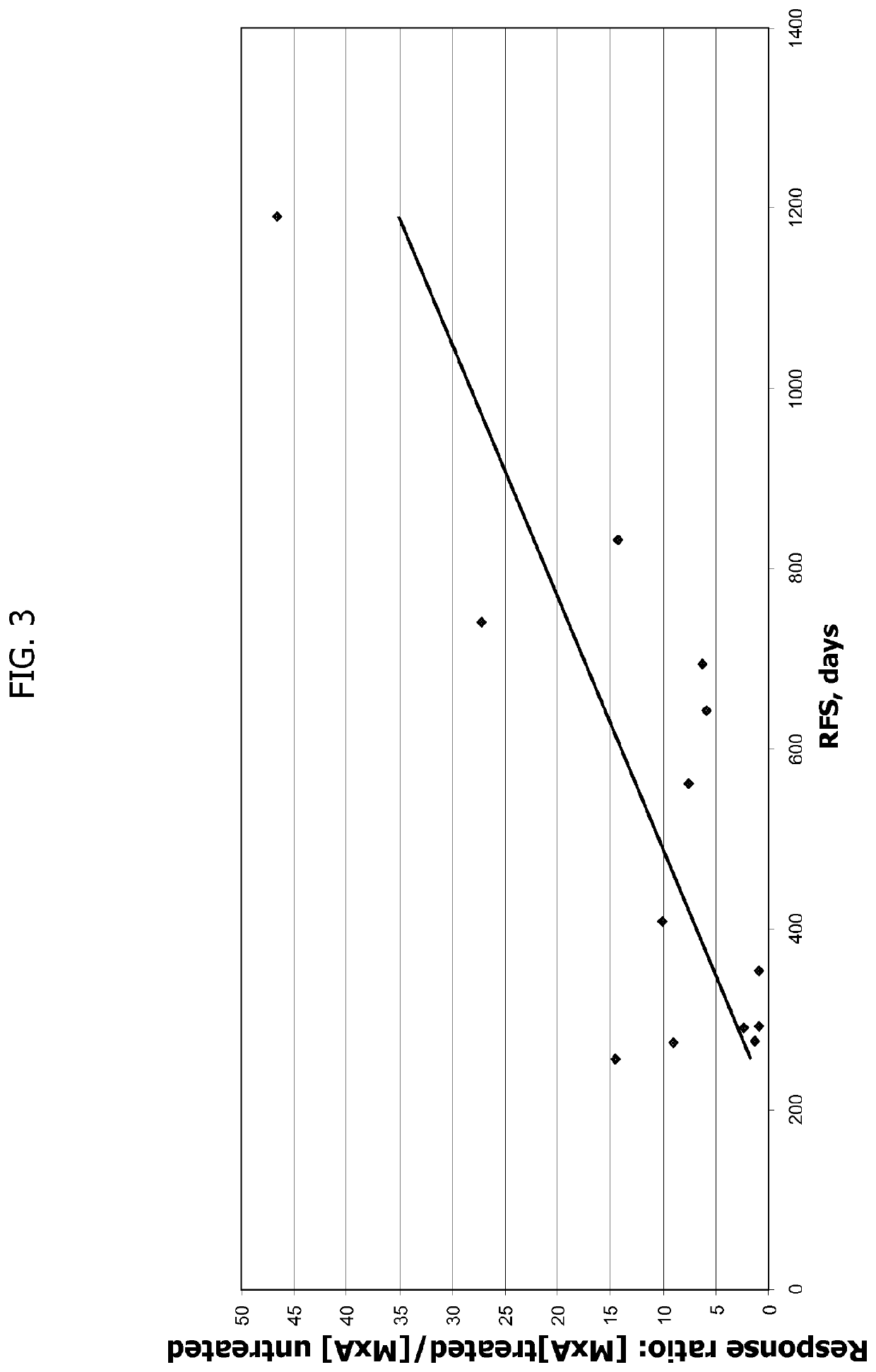
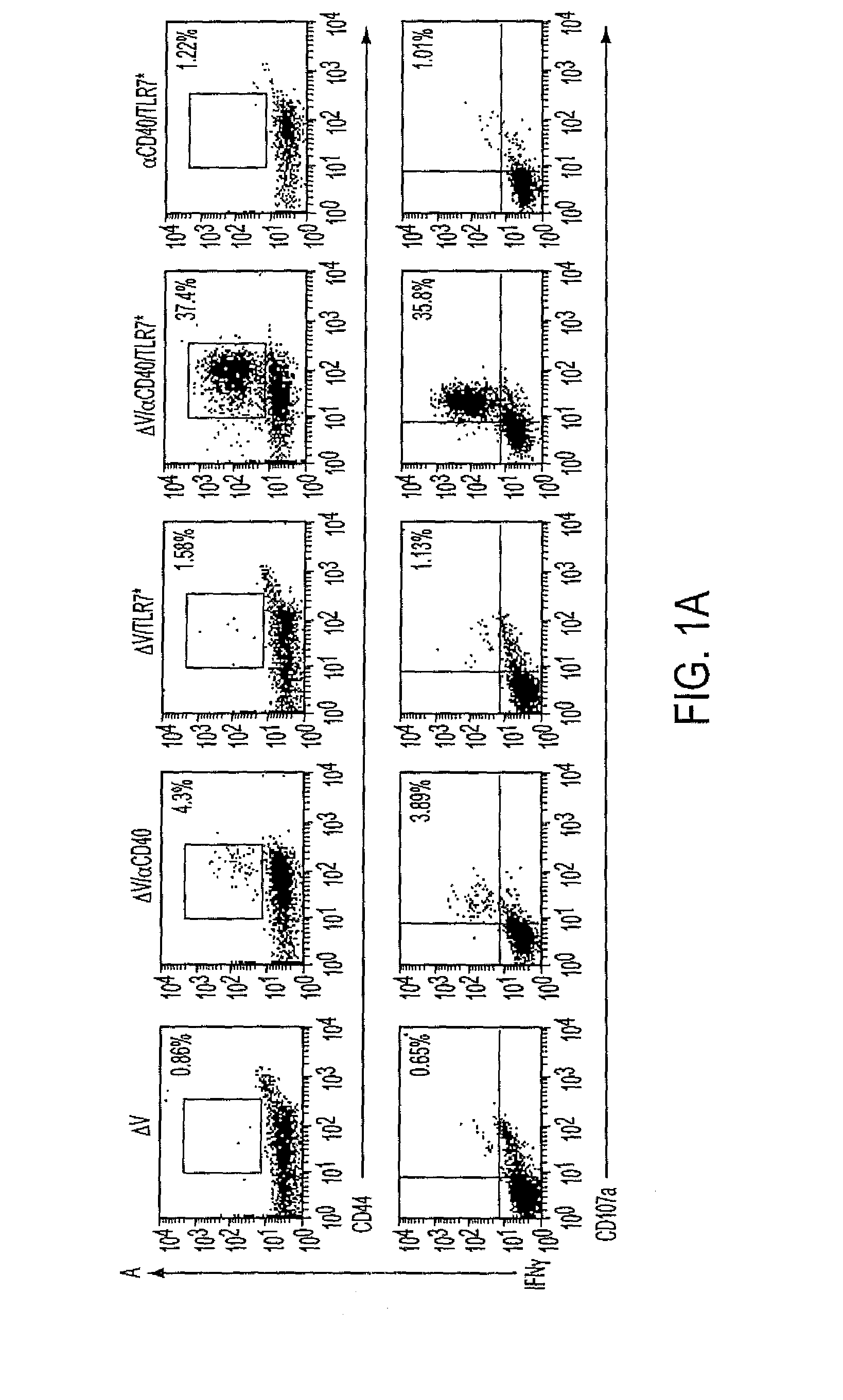
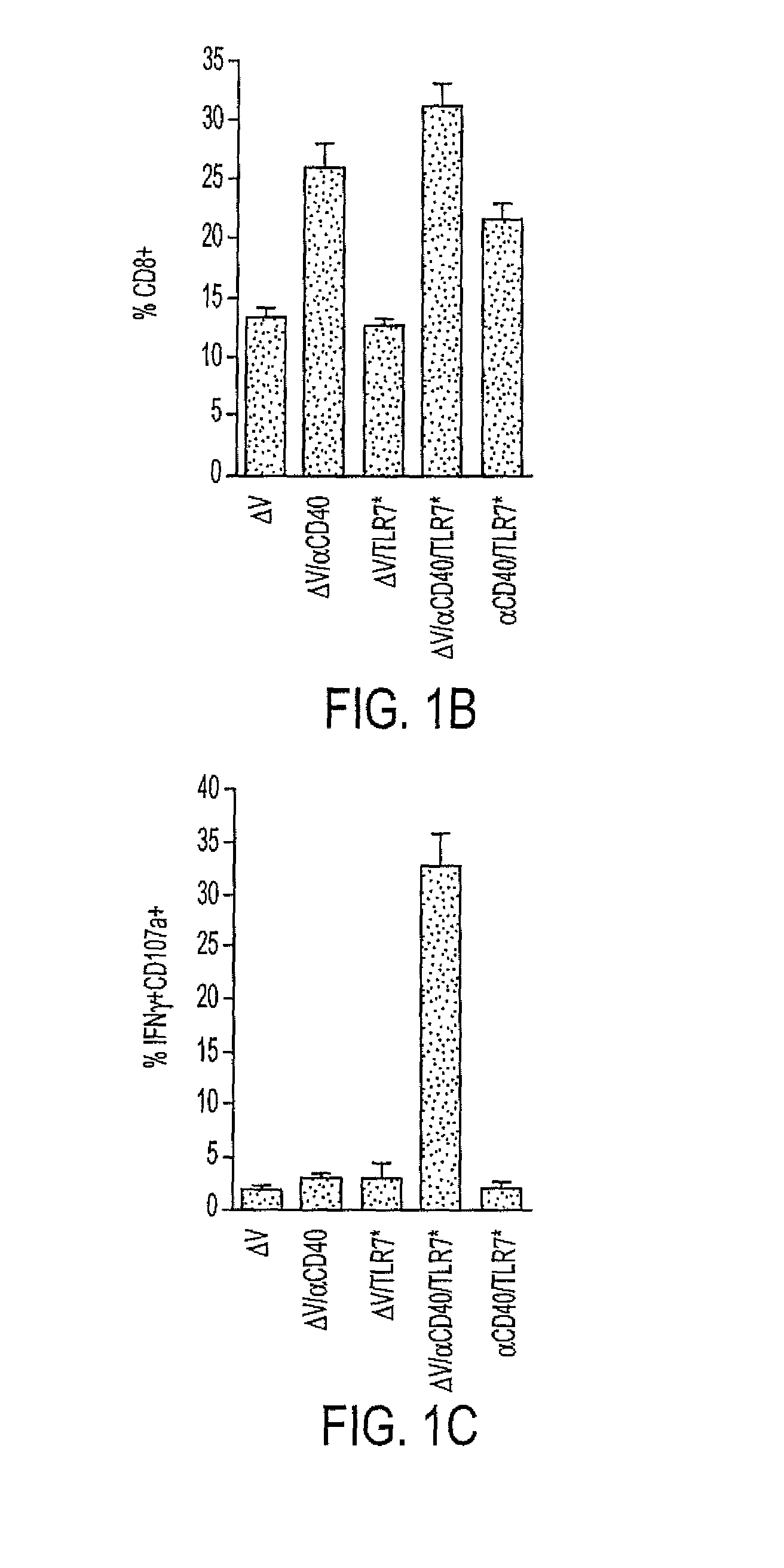
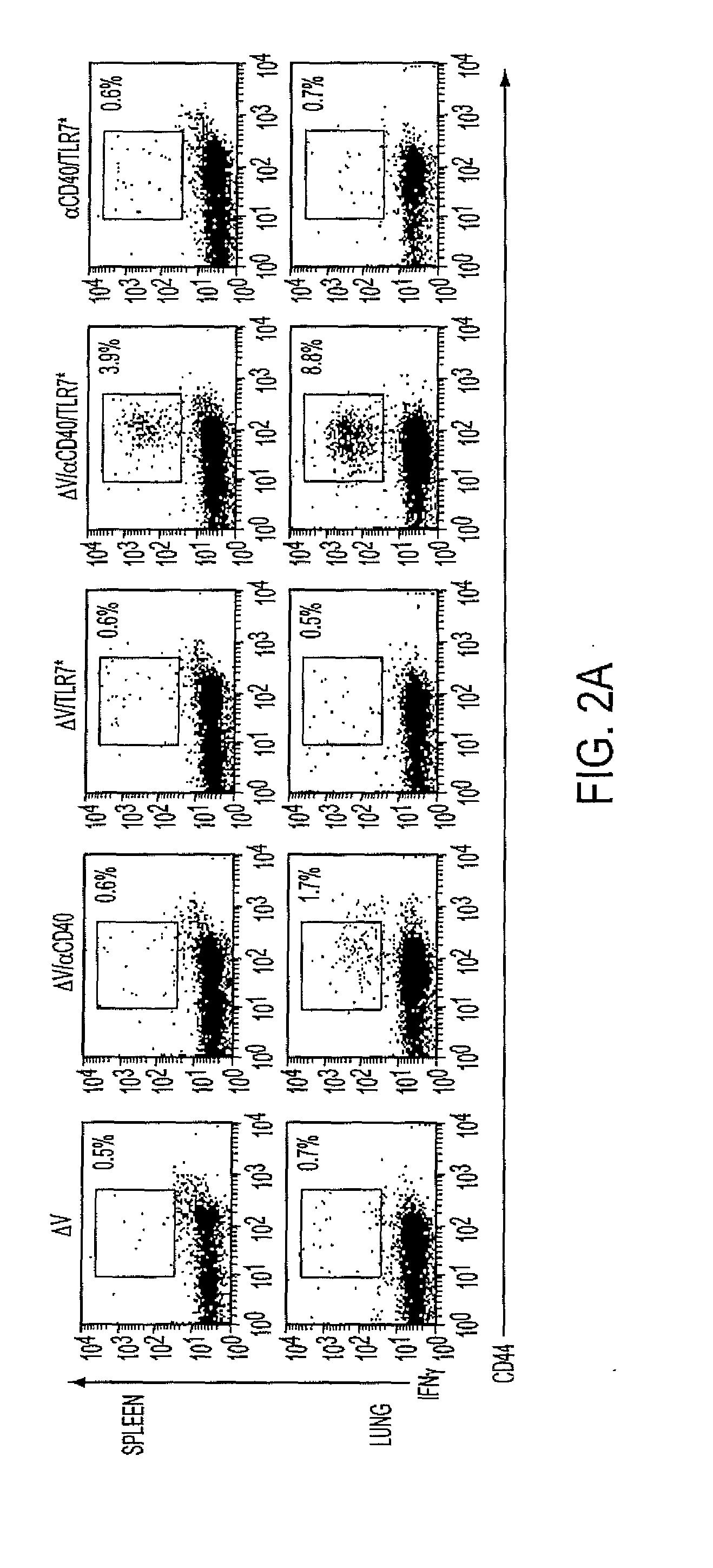
Cd40 agonist antibody/type 1 interferon synergistic adjuvant combination, conjugates containing and use thereof as a therapeutic to enhance cellular immunity
ActiveUS20100317111A1Good curative effectImprove immunityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAgonist
A synergistic adjuvant is provided comprising synergistically effective amounts of at least one type 1 interferon and at least one CD40 agonist, wherein these moieties may be in the same or separate compositions. In addition, fusion proteins and DNA conjugates which contain a type 1 interferon / CD40 agonist / antigen combination are provided. The use of these compositions, protein and DNA conjugates as immune adjuvants for treatment of various chronic diseases such as HIV infection and for enhancing the efficacy of vaccines (prophylactic and therapeutic) is also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
Use of tlr agonists and/or type 1 interferons to alleviate toxicity of tnf-r agonist therapeutic regimens
Improved (safer and more effective) methods of therapy using TNF-R agonists, e.g., CD40 agonists are provided. These methods provide for the addition of an amount of a type 1 interferon and / or a TLR agonist that is effective to prevent or reduce the toxicity (liver toxicity) that may otherwise result in some patients of the TNF-R agonist is used as a monotherapy (without the type 1 interferon and / r TLR agonist).
Owner:IMMURX INC
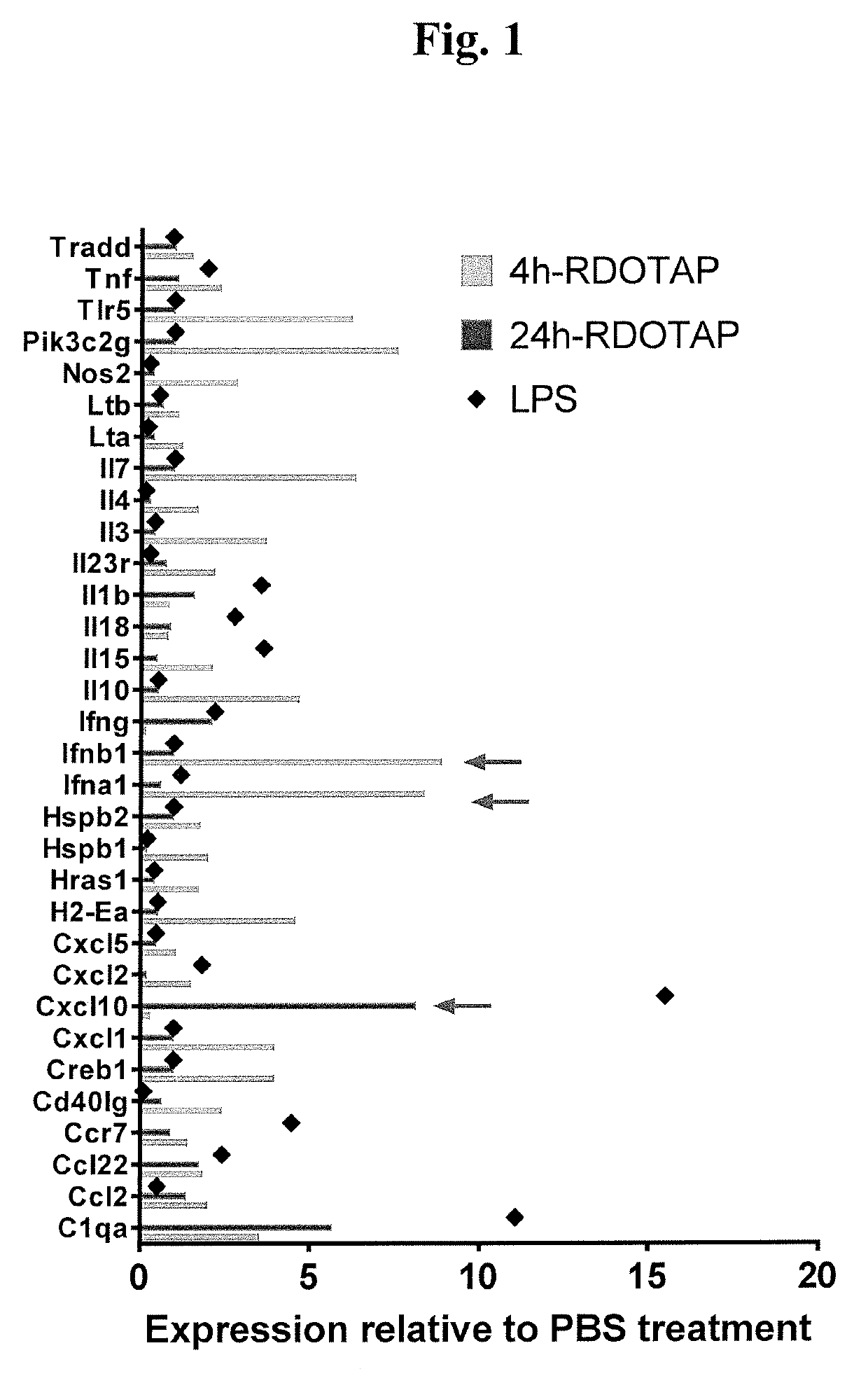
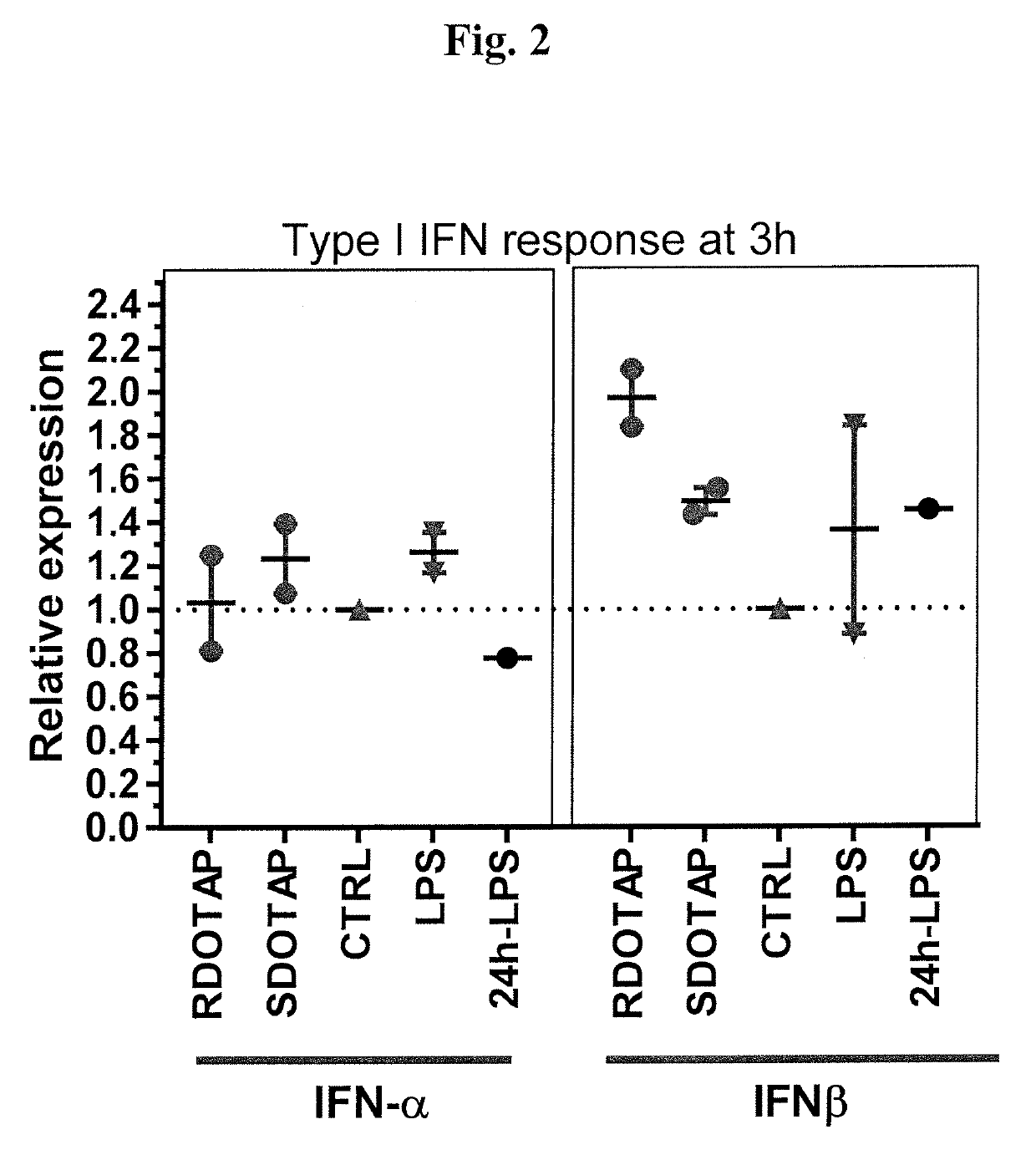
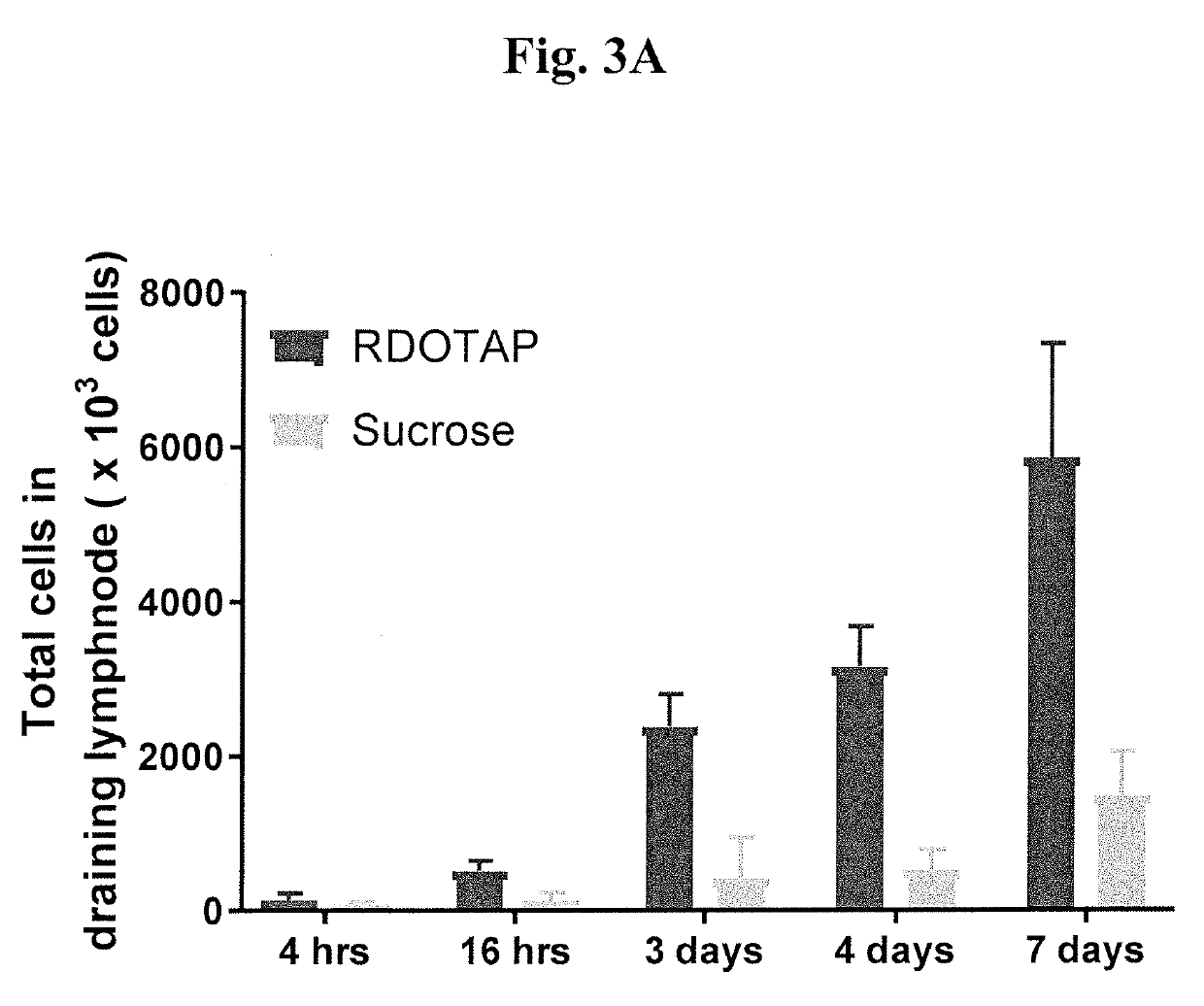
Methods and compositions comprising cationic lipids for stimulating type 1 interferon genes
PendingUS20190321321A1Effective and robust cytotoxic T cell immune responseEffective preventionViral antigen ingredientsCancer antigen ingredientsAntigenLipid formation
Methods and compositions for modifying type I IFN signaling pathways in a subject comprising the administration a cationic lipid to the subject are provided. The cationic lipids comprise 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), N-1-(2,3-dioleoyloxy)-propyl-N,N,N-trimethyl ammonium chloride (DOTMA), 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine (DOEPC), enantiomers and combinations thereof. The compositions may further comprise one or more antigenic components, wherein such components are autologous or nonautologous.
Owner:PDS BIOTECH
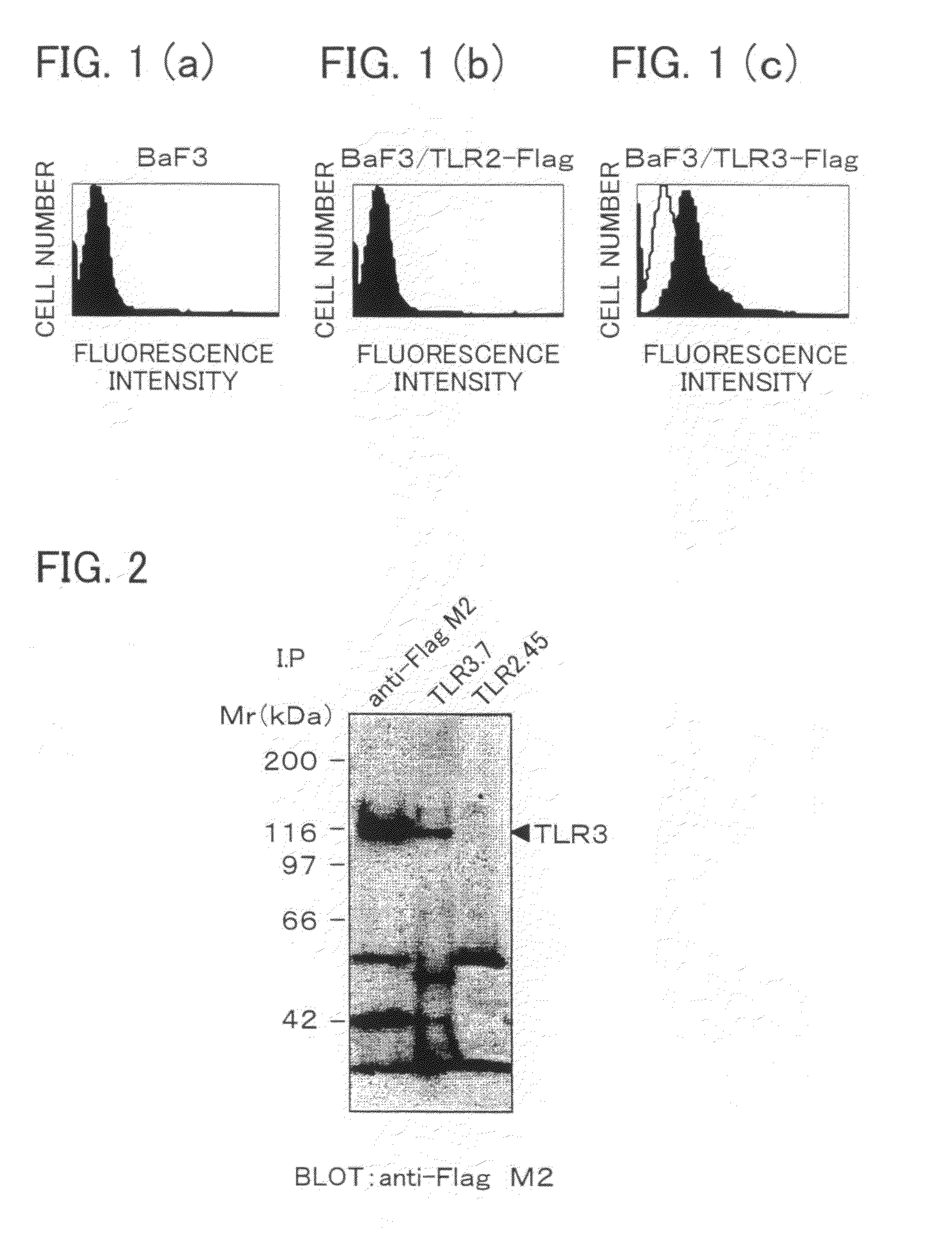
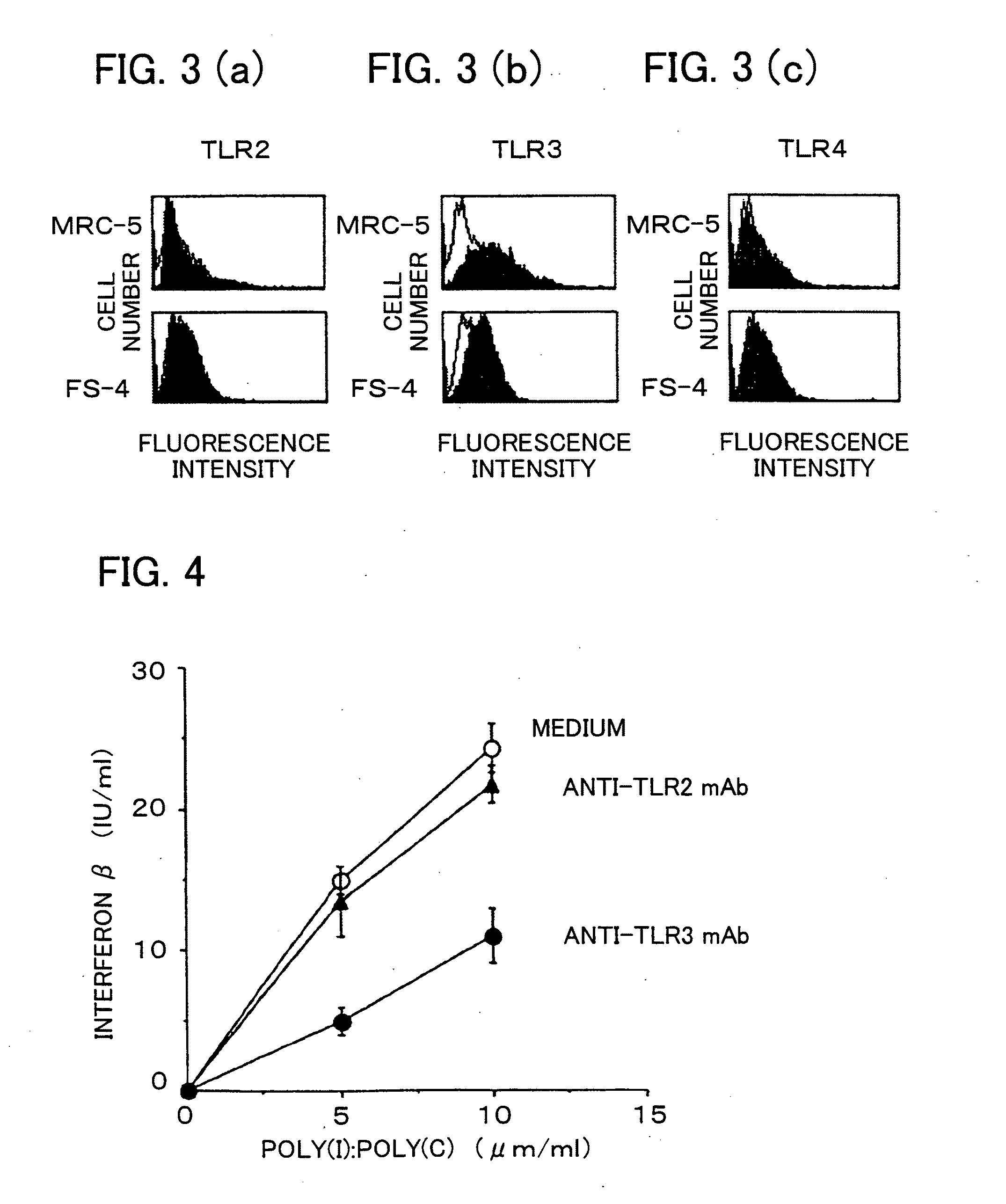
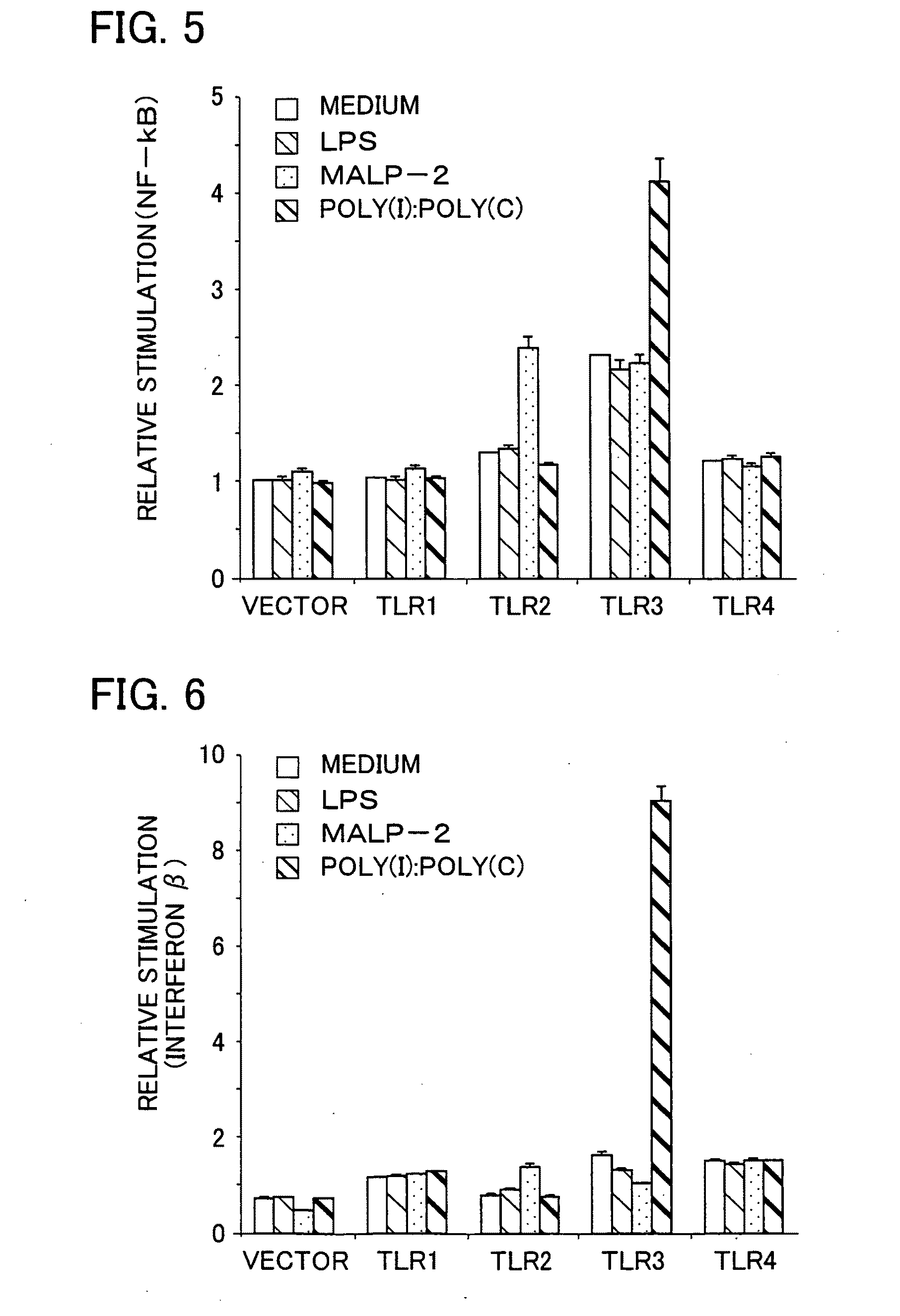
Antibody and inhibitor, and transfection method or kit using them
ActiveUS20090105460A1Suppress immune responseImprove viral infectionImmunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsMonoclonal antibodyToll-like receptor
The present invention provides a monoclonal antibody which specifically binds to human Toll-like receptor 3 and inhibits production of type 1 interferon. It also provides an inhibitor which (a) suppresses a double-stranded RNA-mediated immune response in a cell which expresses Toll-like receptor 3 that recognizes the double-stranded RNA and produces type I interferon, and (b) includes an antibody, which binds to the Toll-like receptor 3 and inhibits production of the type I interferon. Particularly, the monoclonal antibody is against human Toll-like receptor 3. Further, a transfection method and kit are provided. Production of type I interferon can be controlled by using an antibody which specifically binds to Toll-like receptor 3 that recognizes a double-stranded RNA and produces type I interferon.
Owner:JAPAN SCI & TECH CORP
Anti-cd47 combination therapy
PendingCN110087673AOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesAntigen binding
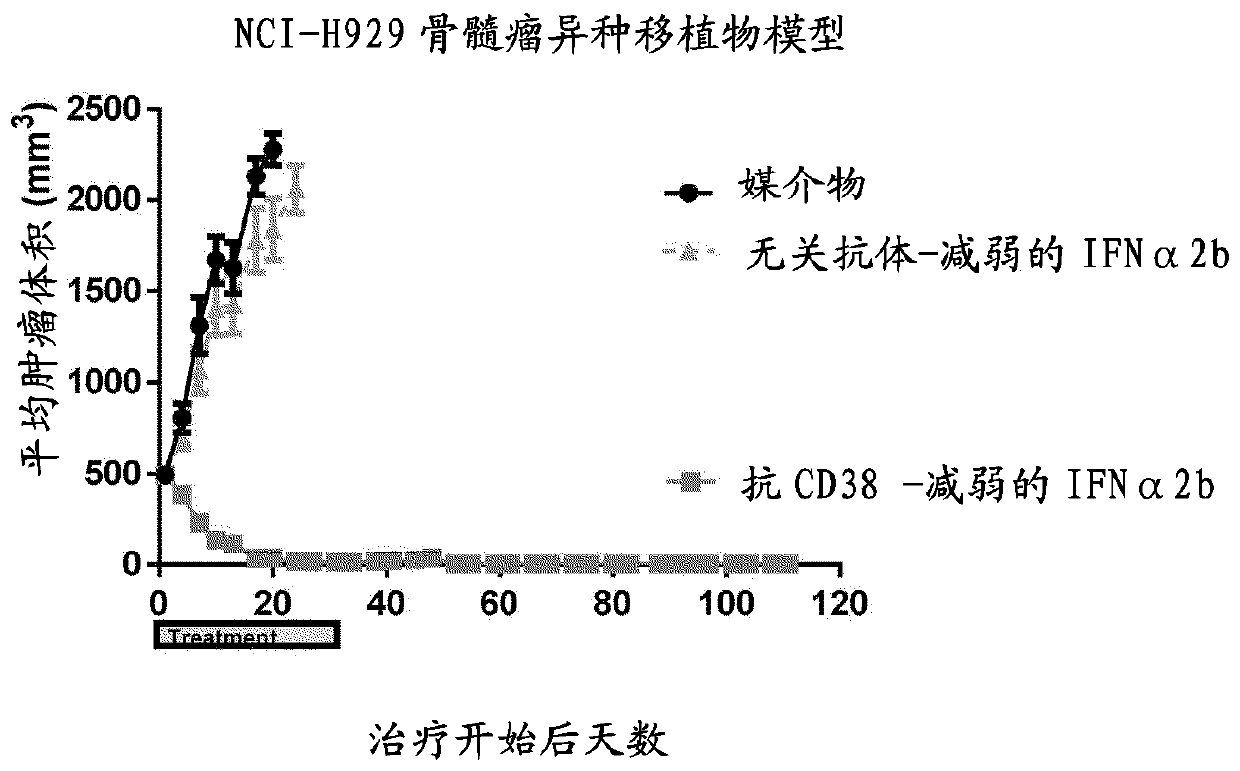
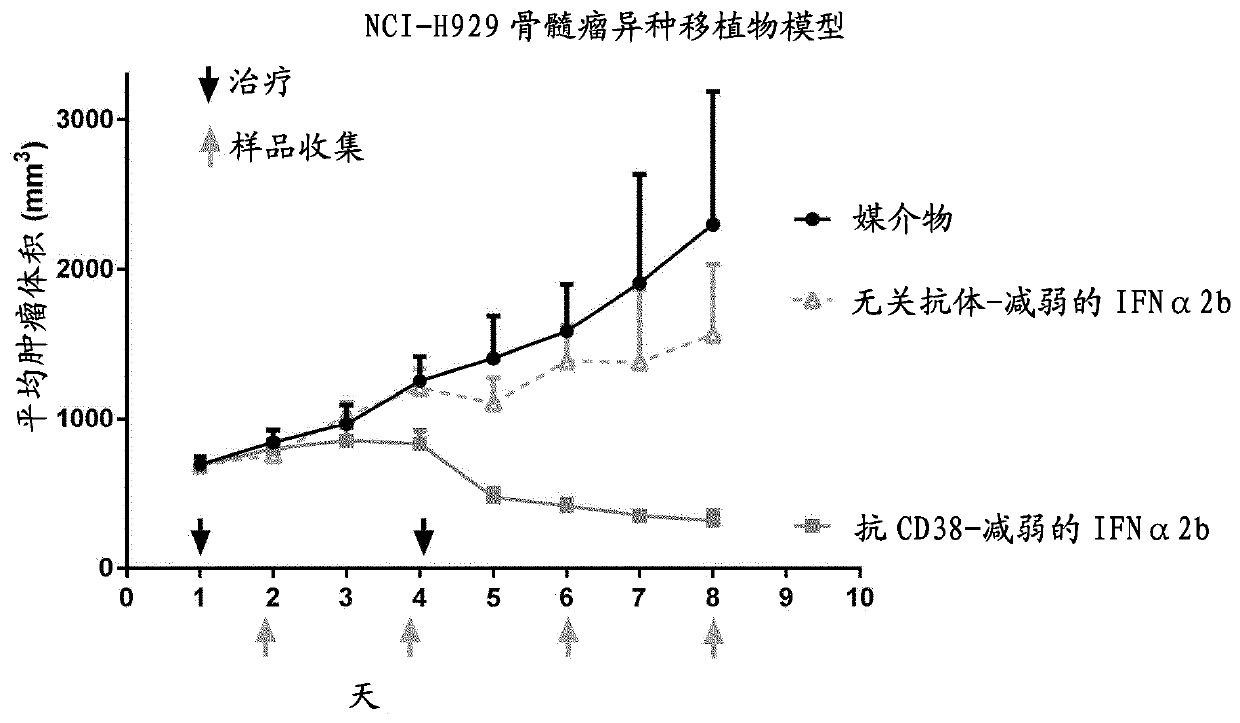
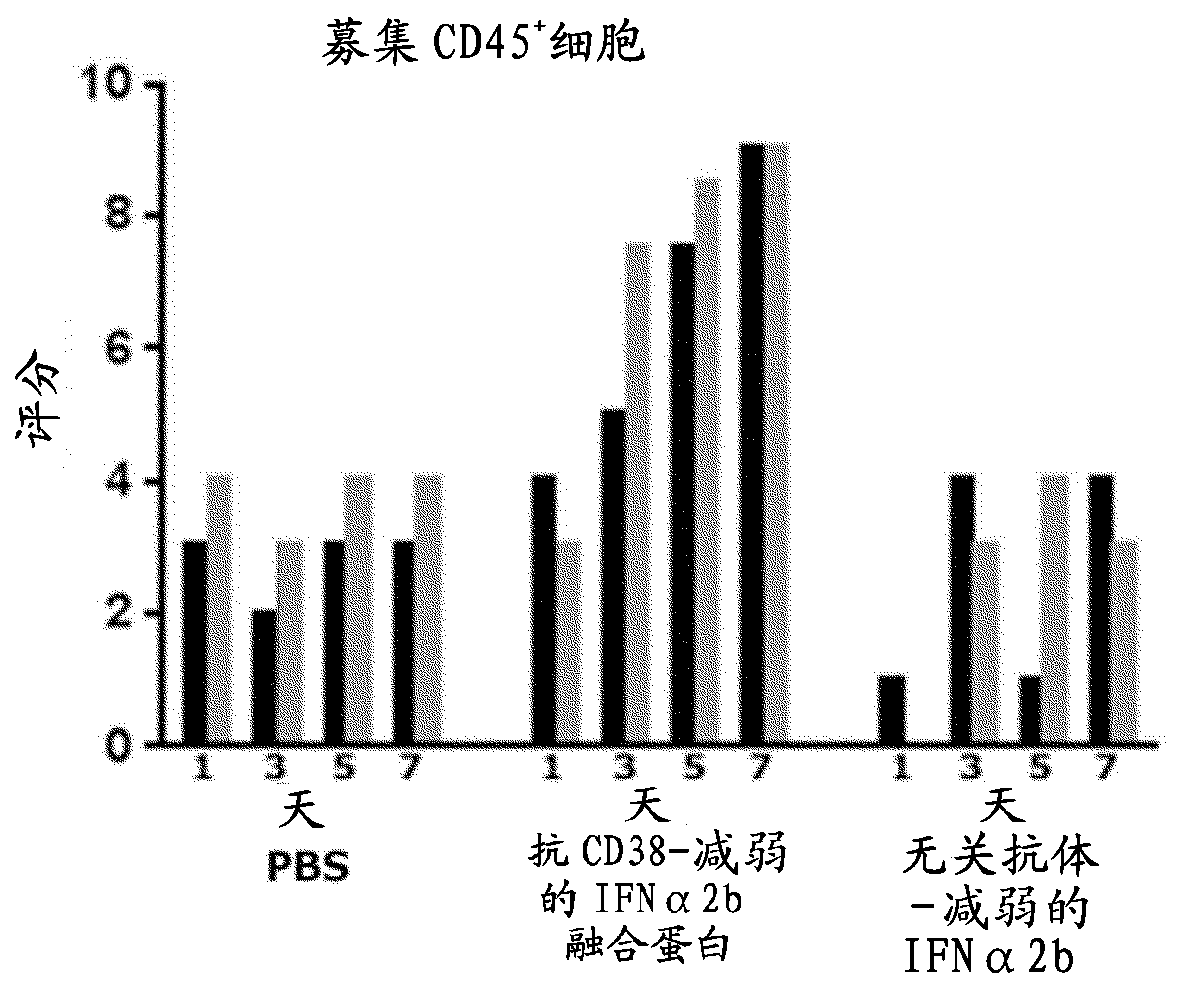
The present invention provides a combination therapy for treating a tumor in a subject. The combination comprises two elements. The first is a polypeptide construct comprising an attenuated Type 1 interferon (IFN) linked to an antibody which binds to a cell surface- associated antigen expressed on the tumour cell and comprising a functional Fc region. The second is a CD47 antagonist which inhibitsthe interaction CD47 with the SIRP alpha receptor.
Owner:TEVA PHARMA AUSTRALIA PTY LTD
Interferon-alpha induced genes
InactiveUS20070117145A1Microbiological testing/measurementDisease diagnosisInterferon therapyInterferon receptor
The present disclosure relates to identification of previously known genes as being genes upregulated by interferon-α administration, in particular the human genes corresponding to the cDNA sequence in GenBank designated g4758303, g5453897, g4505186, g2366751, g33917, g4504962, g3978516, g5924396, g4505656, g1504007, g3702446, g4001802, g292289, g4557226, g4507646 and g4507170. Determination of expression products of these genes is proposed as having utility in predicting responsiveness to treatment with interferon-α and other interferons which act at the Type 1 interferon receptor.
Owner:PHARM PACIFIC PTY LTD
Yeast-based immunotherapy and type i interferon sensitivity
Disclosed are methods of treating individuals with yeast-based immunotherapy who have been preselected as being sensitive to type I interferons, as well as methods for selecting individuals for treatment with yeast-based immunotherapeutic compositions and methods for enhancing or improving an individual's response to yeast-based immunotherapy, based on the individual's sensitivity to type 1 interferons (T1IFNs).
Owner:UNIV OF COLORADO THE REGENTS OF
Yeast-based immunotherapy and type I interferon sensitivity
Disclosed are methods of treating individuals with yeast-based immunotherapy who have been preselected as being sensitive to type I interferons, as well as methods for selecting individuals for treatment with yeast-based immunotherapeutic compositions and methods for enhancing or improving an individual's response to yeast-based immunotherapy, based on the individual's sensitivity to type 1 interferons (T1IFNs).
Owner:UNIV OF COLORADO THE REGENTS OF
Use of tlr agonists and/or type 1 interferons to alleviate toxicity of tnf-r agonist therapeutic regimens
Improved (safer and more effective) methods of therapy using TNF-R agonists, e.g., CD40 agonists are provided. These methods provide for the addition of an amount of a type 1 interferon and / or a TLR agonist that is effective to prevent or reduce the toxicity (liver toxicity) that may otherwise result in some patients of the TNF-R agonist is used as a monotherapy (without the type 1 interferon and / or TLR agonist).
Owner:NOELLE RANDOLPH J +2
Yeast-based immunotherapy and type i interferon sensitivity
Disclosed are methods of treating individuals with yeast-based immunotherapy who have been preselected as being sensitive to type I interferons, as well as methods for selecting individuals for treatment with yeast-based immunotherapeutic compositions and methods for enhancing or improving an individual's response to yeast-based immunotherapy, based on the individual's sensitivity to type 1 interferons (T1IFNs).
Owner:UNIV OF COLORADO THE REGENTS OF
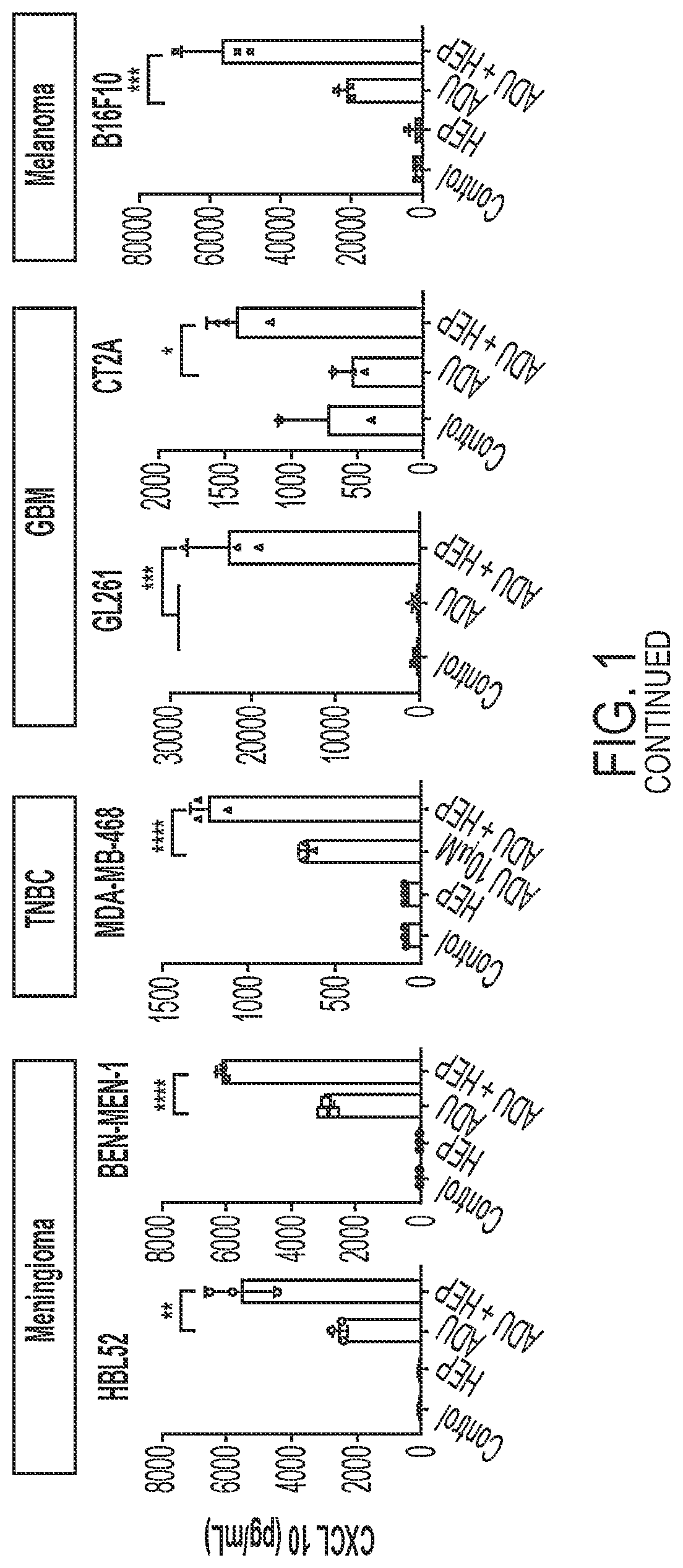
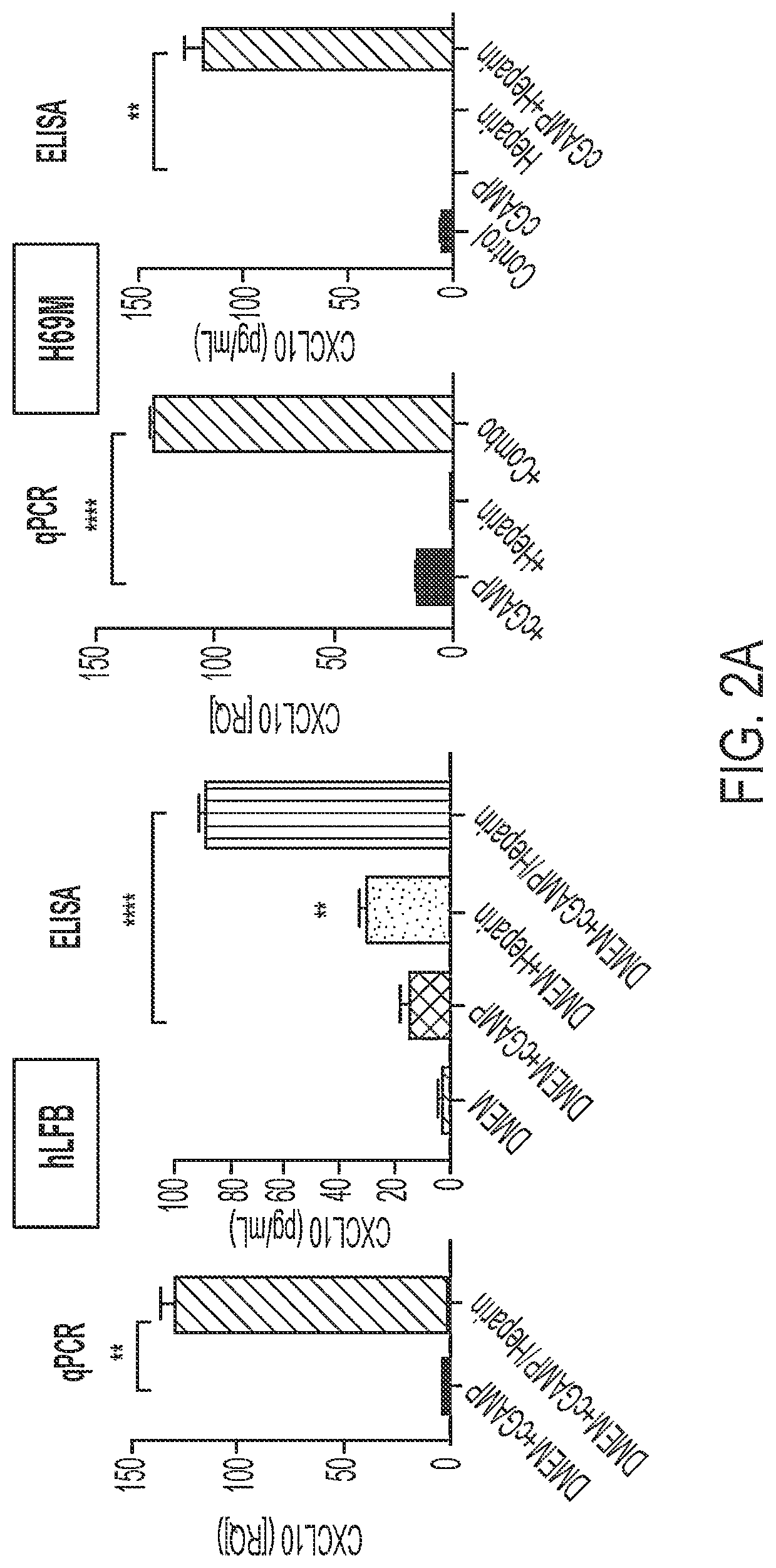
Use of heparin to promote type 1 interferon signaling
PendingUS20220305048A1Improve the situationShorten the progressOrganic active ingredientsPeptide/protein ingredientsPharmaceutical drugPolysaccharide
Disclosed herein are methods for treating a subject having cancer by coadministering a stimulator of interferon signaling and a heparin polysaccharide. Also disclosed herein are pharmaceutical compositions that include a stimulator of interferon signaling and a heparin polysaccharide.
Owner:DANA FARBER CANCER INST INC
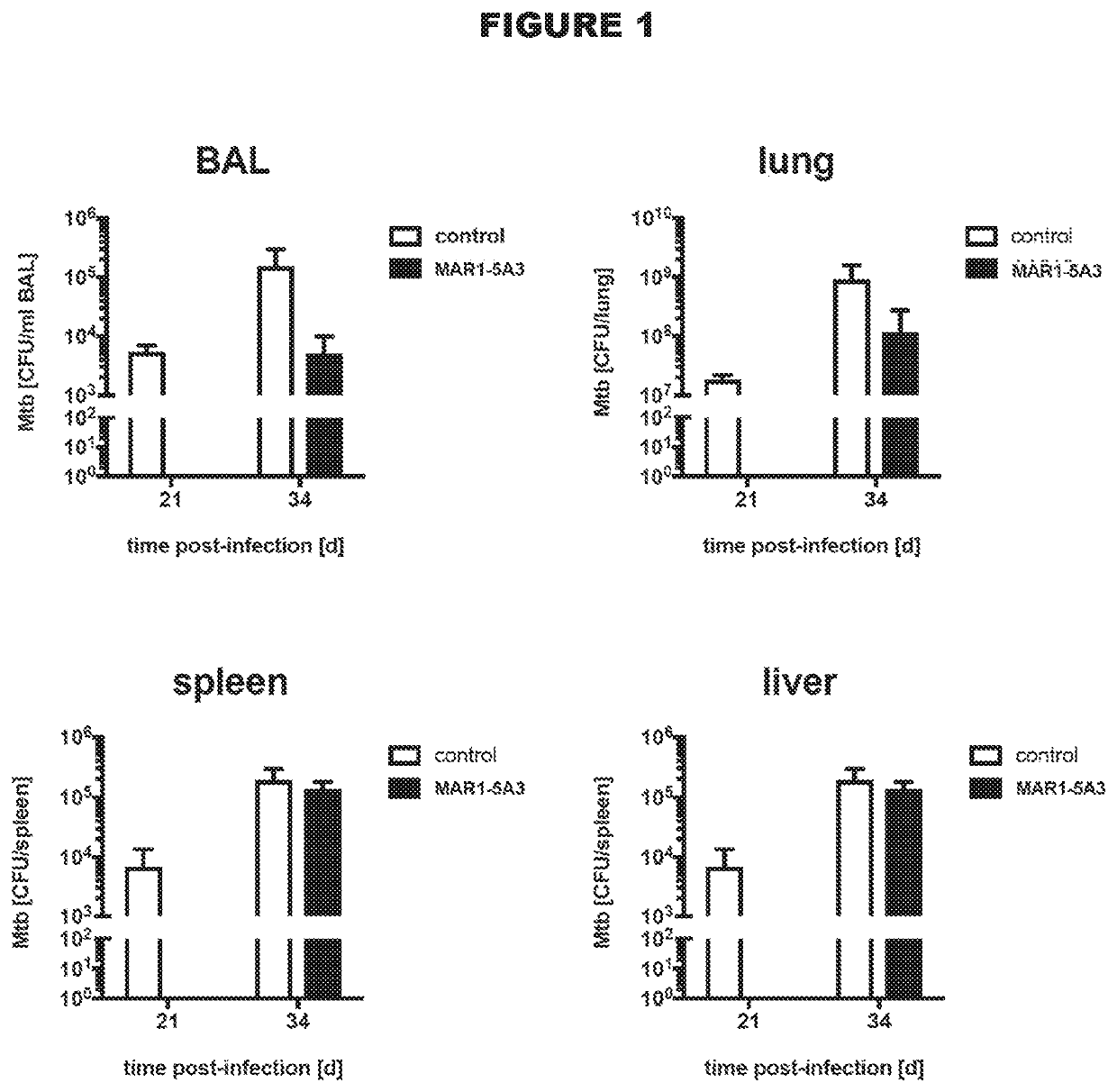
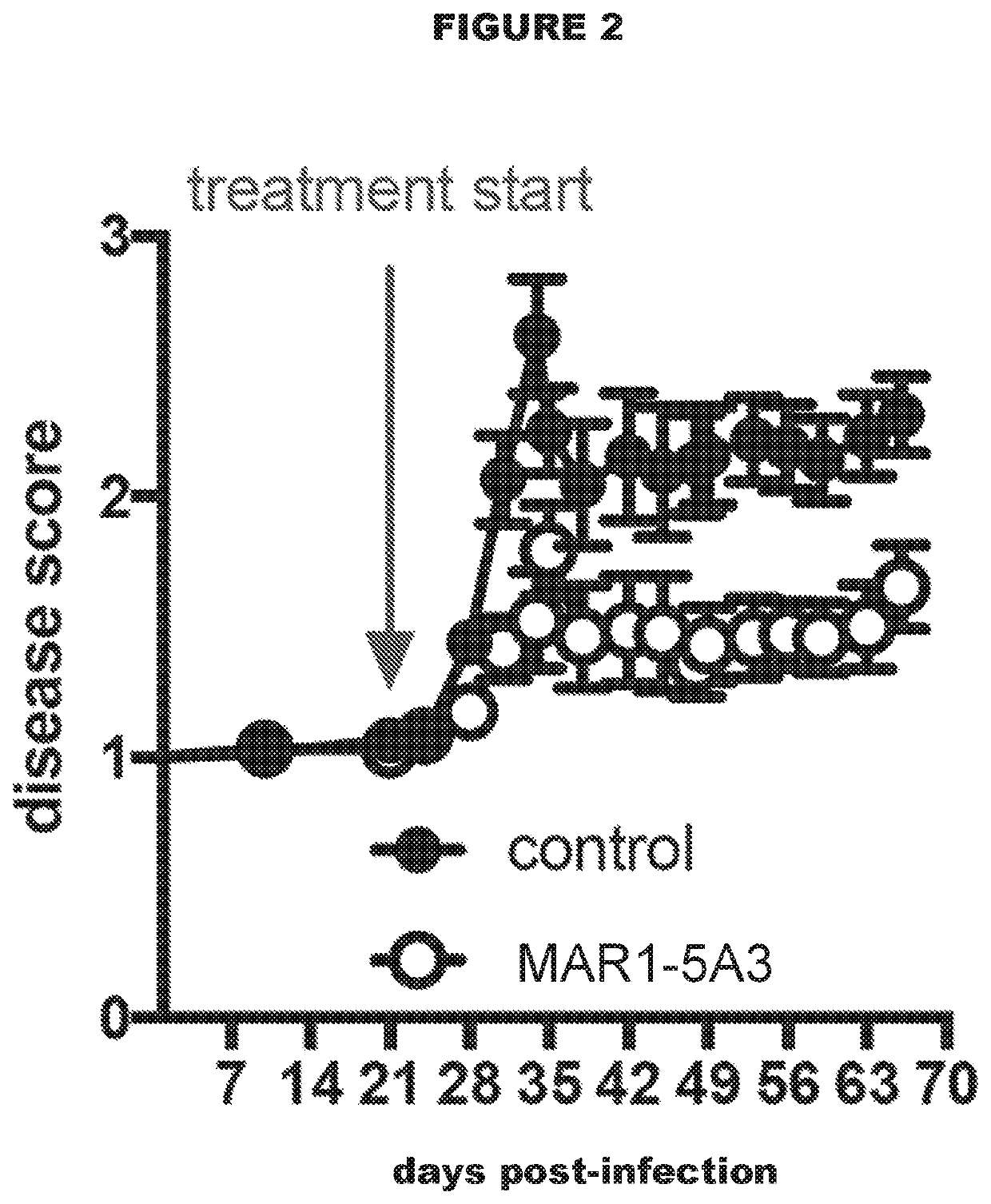
Type 1 interferon receptor antagonists for use in methods of treating tuberculosis and other infectious diseases
InactiveUS20200031943A1Antibacterial agentsImmunoglobulins against cell receptors/antigens/surface-determinantsInfectious DisorderLeishmaniasis
The present invention relates to the use of type 1 interferon receptor antagonists that specifically bind IFNAR1 and / or IFNAR2 and inhibit type 1 IFNs signalling in methods of treating tuberculosis and other infectious diseases, in which type 1 interferon signalling has been found to be deleterious. Infectious diseases that can be treated according to the present invention include tuberculosis and leishmaniasis.
Owner:AUSTRIANNI GMBH
Method for producing soluble recombinant interferon protein without denaturing
The present invention relates to the field of recombinant protein production in bacterial hosts. It further relates to extraction of soluble, active recombinant protein from an insoluble fraction without the use of denaturation and without the need for a refolding step. In particular, the present invention relates to a production process for obtaining high levels a soluble recombinant Type 1 interferon protein from a bacterial host.
Owner:PELICAN TECH HLDG INC
Pharmaceutical composition for cancer treatment
The invention relates to therapeutic compositions for the treatment of cancer and, more specifically, compositions containing an agonist ligand for receptor 4-1BB and a type-1 interferon, the simultaneous or sequential delivery of which results in a synergic antitumour effect in relation to the individual delivery of any of the components. The invention also relates to the therapeutic uses of the combinations of the invention for the treatment of cancer. The invention further relates to polynucleotides that code for compounds, vectors and cells containing same, as well as to the use thereof for the treatment of cancer.
Owner:生物医学工程应用医药研究中心有限公司 +1
Interferon-alpha induced gene
InactiveUS20050265967A1Prediction of responsivenessCytokine-induced proteinsPeptide/protein ingredientsInterferon therapyInterferon receptor
The present invention relates to identification of a gene upregulated by interferon-administration corresponding to the cDNA sequence set forth in SEQ. ID. No. 1. Determination of expression products of this gene is proposed as having utility in predicting responsiveness to treatment with interferon- and other interferons which act at the Type 1 interferon receptor. Therapeutic use of the protein encoded by the same gene is also envisaged.
Owner:PHARM PACIFIC PTY LTD
Vaccine adjuvants
PendingUS20210187104A1Interferon response is increasedStimulating innate immune responseOrganic active ingredientsImmunological disordersImmunity responseImmunogenicity
The present application relates to an adjuvant which is suitable to be used in vaccines or other immunogenic compositions. Specifically, the adjuvant promotes the induction of interleukin-1 (IL-1), type 1 interferons (IFNs), such as IFNα, and IFNβ, type 2 interferons, such as IFNγ and / or tumour necrosis factor (TNF) response, such as TNFα, and elicits or enhances an immune response, preferably in neonatal, juvenile or paediatric animal and / or human populations.
Owner:TRINITY COLLEGE DUBLIN +1
Interferon-alpha induced gene
InactiveUS20070226815A1Prediction of responsivenessAntibacterial agentsOrganic active ingredientsInterferon therapyInterferon receptor
The present invention relates to identification of a gene upregulated by interferon-a administration corresponding to the cDNA sequence set forth in SEQ. ID. NO. 1. Determination of expression products of this gene is proposed as having utility in predicting responsiveness to treatment with interferon-α and other interferons which act at the Type 1 interferon receptor. Therapeutic use of the protein encoded by the same geneis also envisaged.
Owner:PHARM PACIFIC PTY LTD
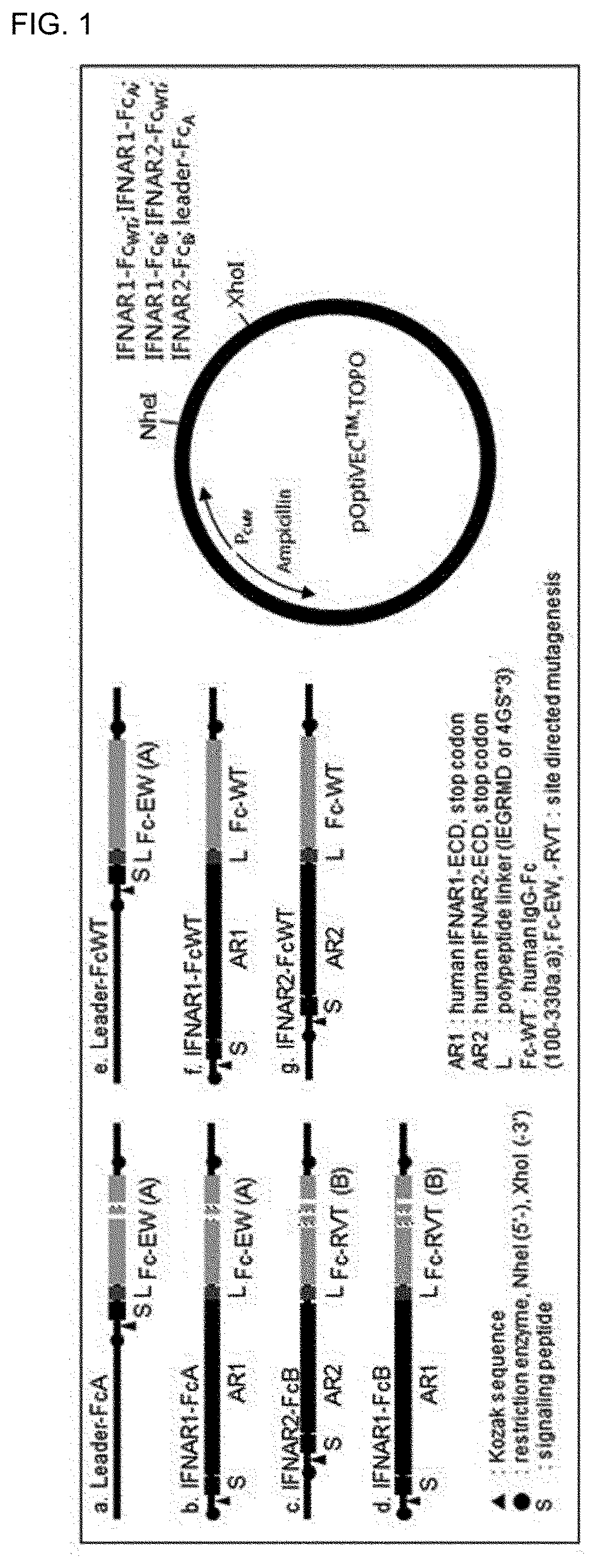
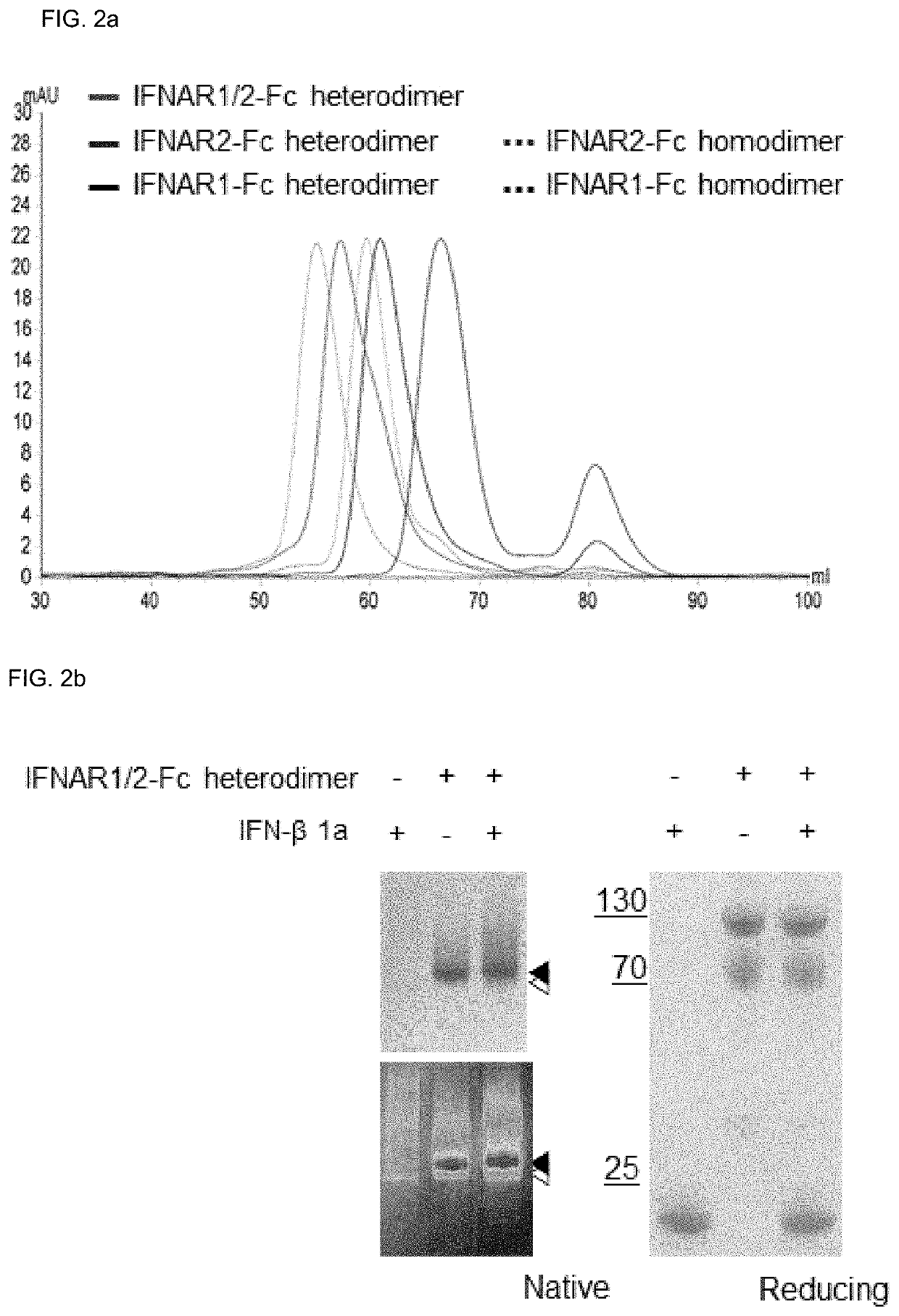
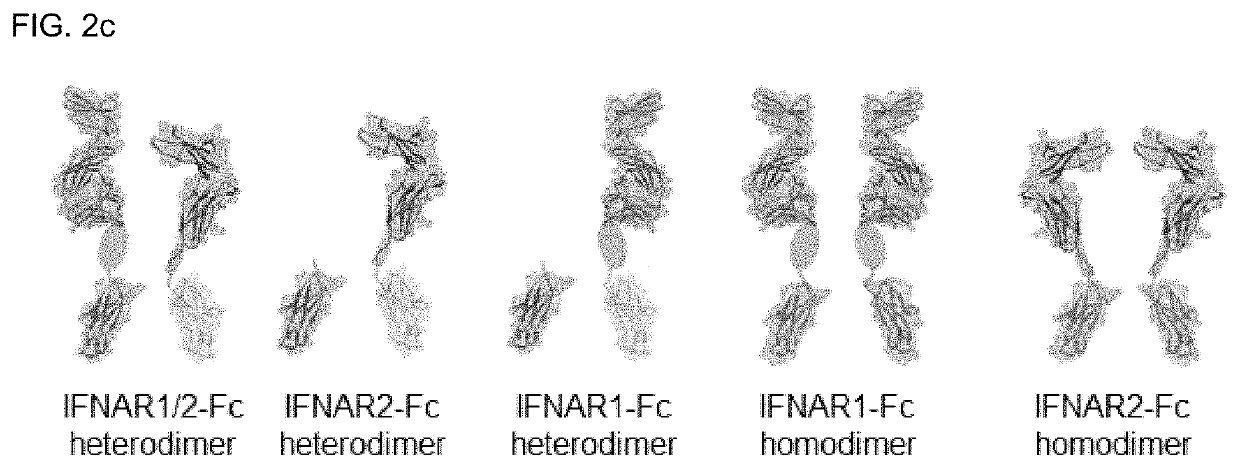
Type 1 interferon neutralizing fc-fusion protein and use thereof
PendingUS20220089684A1Improve biological activityGood initiativePeptide/protein ingredientsAntibody mimetics/scaffoldsDimerDisease
The present invention relates to a type 1 interferon neutralizing FC-fusion protein and a use thereof and, more specifically, to: a dimer-type polypeptide to which a monomer comprising an interferon receptor fragment or an antibody Fc fragment is bound; a preparation method there for; and a pharmaceutical composition comprising same. The type 1 interferon neutralizing FC-fusion protein of the present invention blocks binding between type 1 interferon and an interferon receptor, and has an excellent ability of inhibiting the signaling and biological activities of interferon, thereby enabling diseases mediated by a type 1 interferon to be effectively treated.
Owner:SEOUL NAT UNIV R&DB FOUND +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com