Anti-cd47 combination therapy
A combination therapy, antibody technology, applied in the field of combination therapy for tumor treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
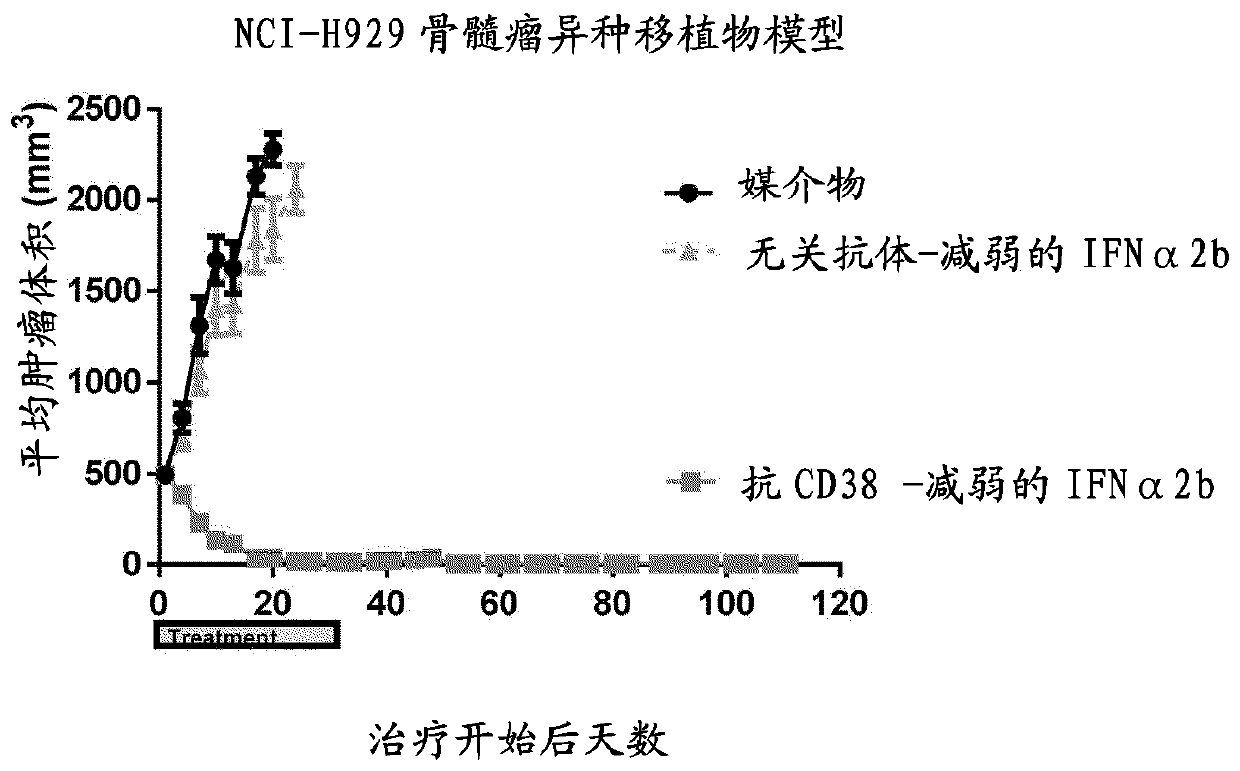
[0146] Robust and durable antitumor activity of CD38-weakened IFNα2b in a myeloma xenograft model
[0147] NCI-H929 plasma cell myeloma cells were maintained in exponentially growing suspension cultures in standard growth media and conditions. Tumor cells for implantation were harvested during log phase growth and grown at 1 x 10 8 Cells / mL were resuspended in 50% Matrigel (BD Biosciences). Will 1×10 7 Two tumor cells (0.1 mL of cell suspension) were subcutaneously implanted into the left flank of 8–9-week-old female severe combined immunodeficiency (SCID) mice. In this model, CD38+ myeloma tumor cells grow as vascularized subcutaneous masses. Tumors were allowed to grow to a mean volume of 150 mm before treatment initiation 3 . Tumors were measured with two-dimensional calipers to monitor size. Mice were treated intraperitoneally in a fixed volume of 0.2 mL with 5 mg / kg anti-CD38-attenuated IFNα2b fusion protein (SEQ ID NOS 507 / 508) or an isotype control fusion protein ...
Embodiment 2
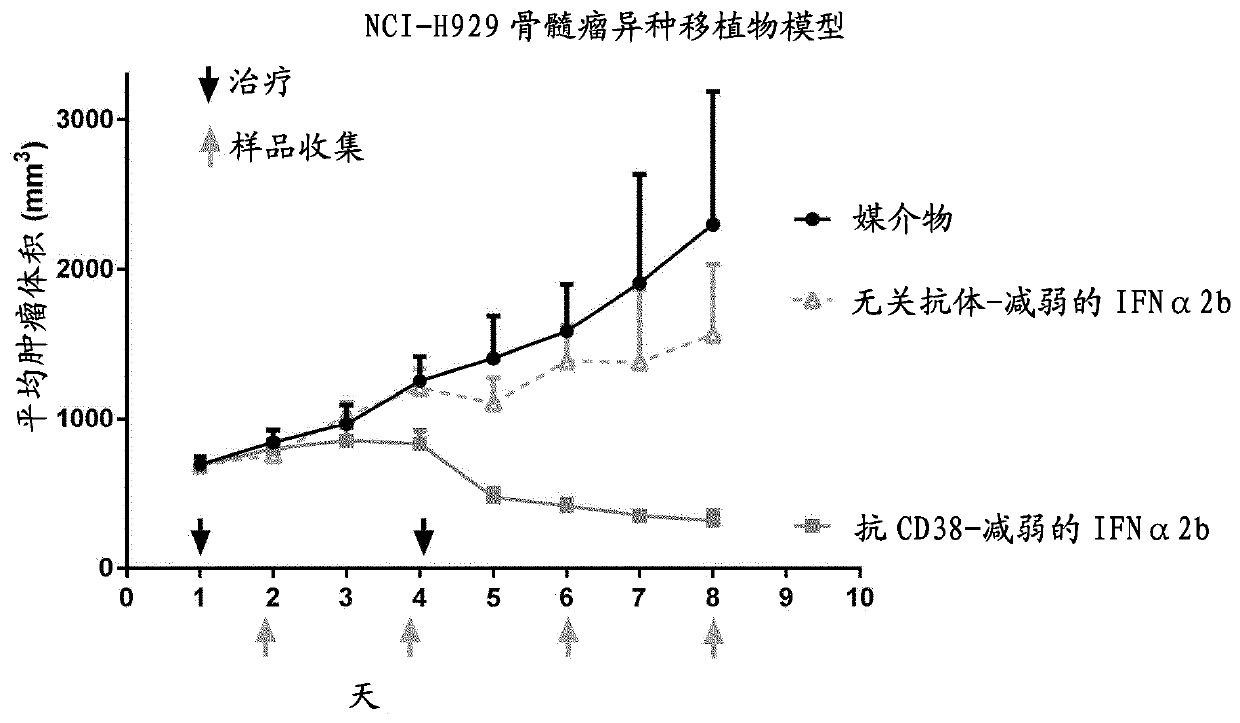
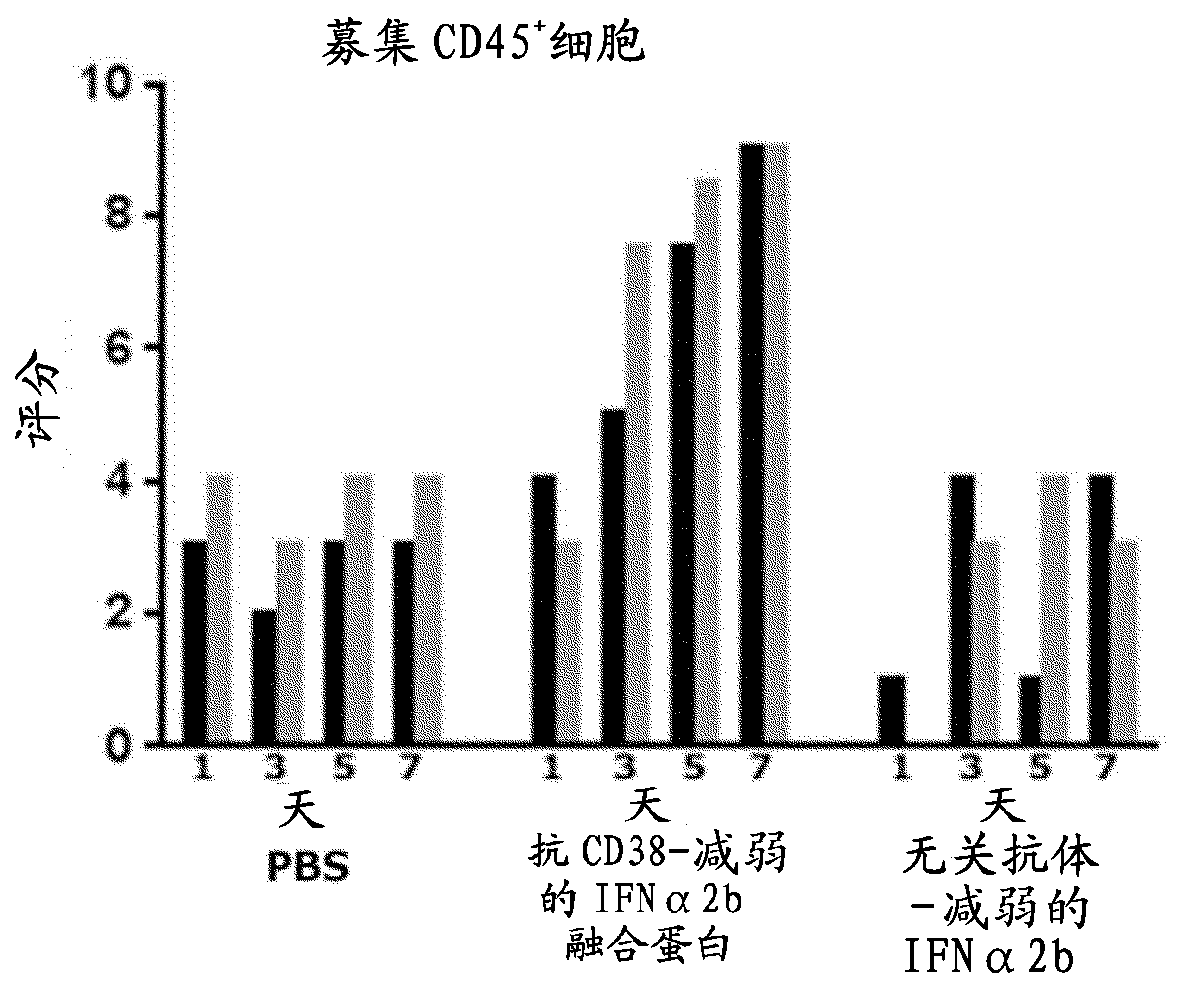
[0149] Involvement of macrophages in anti-CD38-attenuated interferon α2b fusion protein activity in reactive myeloma model H929
[0150] In order to analyze the mechanism mediating the strong anti-tumor activity of the anti-CD38-weakened IFNα2b (h10A2-hIgG4) fusion protein (SEQ ID NOS 507 / 508) described in Example 1, excised from the treated group and the untreated group or control Tumors in groups of mice were evaluated by immunohistochemistry. Cells were grown as described in Example 1, prepared and implanted into mice. Tumors were allowed to grow to an average volume of 600-750mm prior to treatment initiation 3 . Small mice were treated intraperitoneally on days 1 and 4 with PBS, 10 mg / kg anti-CD38-weakened IFNα2b fusion protein (SEQ ID NOS:507 / 508) or an isotype control consisting of an irrelevant antibody fused to attenuated IFNα2b. Rats (12 / group) ( Figure 2A black arrow above the middle x-axis). Tumor size was measured daily and at Figure 2A Mean (+ / -SEM) tumor ...
Embodiment 3
[0155] Involvement of macrophages in anti-human HLA-attenuated interferon α2b fusion protein activity in the non-responsive renal cell carcinoma model 786-0
[0156] The role of macrophage infiltration in a non-responsive xenograft tumor model was investigated. This study was performed in a similar manner to the multiple myeloma xenograft model described in Example 1 and Example 2. Ten million human HLA-expressing 786-0 renal cell carcinoma cells were implanted with Matrigel into SCID mice and the tumors grew to an average volume of 500 mm 3 . exist Figure 3A Mice were treated with PBS or 10 mg / kg anti-HLA-attenuated IFNα2b (HB95-IgG4) fusion protein (SEQ ID NOs: 521 and 522) at time points indicated by black arrows in . The anti-HLA antibodies used in this fusion protein are human-specific and thus bind to human tumor cells but not to any murine cells. Tumors of three mice per group were resected at time points indicated by gray arrows. Immunohistochemical analysis and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com