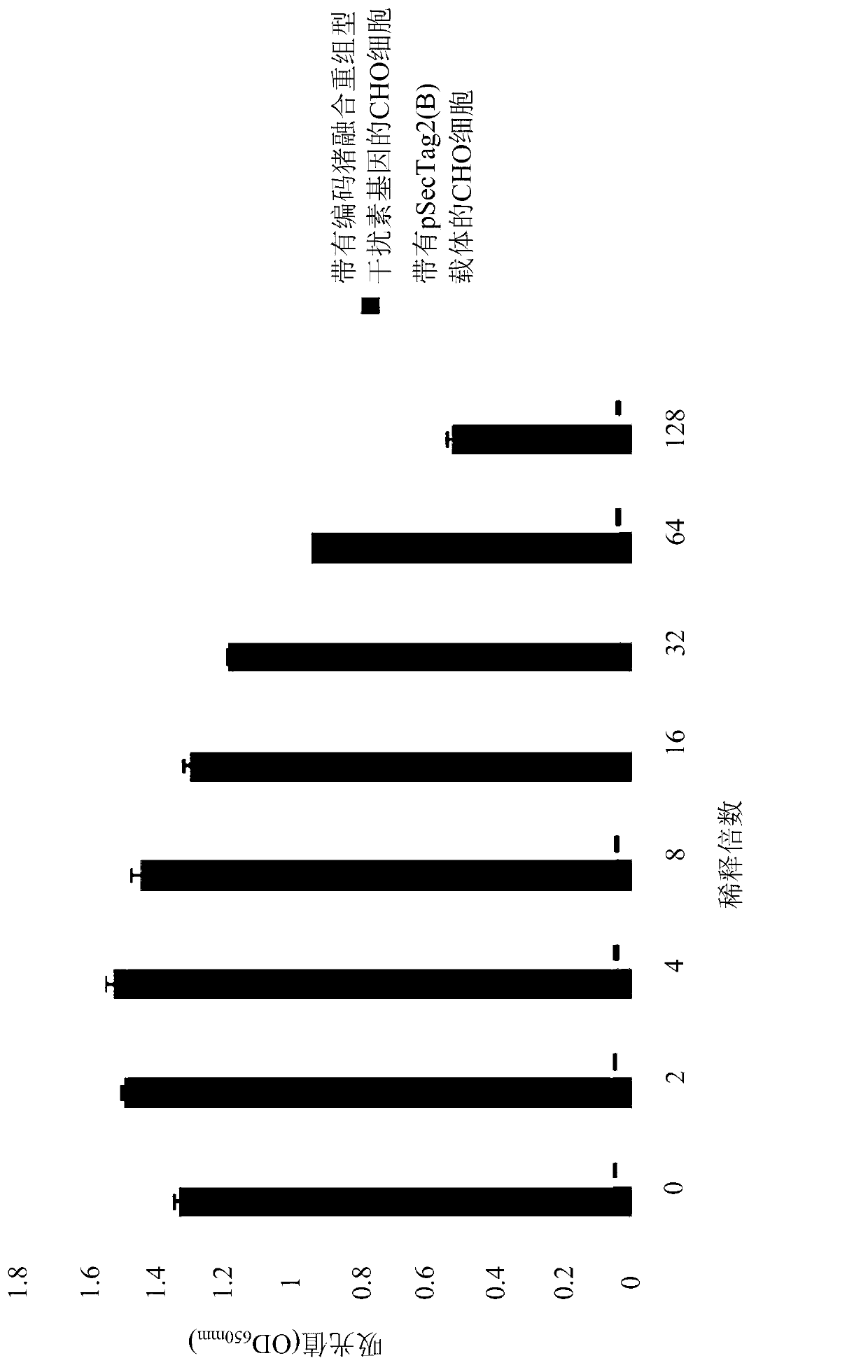
Animal fusion recombinant interferon
An interferon, animal technology, applied in the direction of interferon, fusion polypeptide, cytokine/lymphokine/interferon, etc., can solve the problems of animal husbandry loss, poor effect and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 porcine interferon alpha 1 gene selection
[0029] Peripheral blood mononuclear cells (PBMC) were isolated from the blood of the three-strain hybrid pigs (L×Y-D), and total RNA was extracted by the guanidine thiocyanate (GTC) method. Then the extracted total RNA was subjected to reverse transcription polymerase chain reaction (reverse polymerase chain reaction, RT-PCR). 25mM dNTP, 1μl 3' end complementary primer, 11μl distilled water, 0.5μl RNasin and 0.5μl AMV reverse transcriptase reaction tubes were placed at 42°C for 30 minutes for cDNA synthesis; then the synthesized cDNA was subjected to polymerase chain reaction (polymerase chain reaction, PCR) to proliferate porcine interferon α1 gene (P IFNα1), add 10 μl cDNA, 5 μl 10x PCR reaction solution, 8 μl 1.25 mM dNTP, 1 μl 5’ end forward primer, 1 μl 3’ end reverse primer, 24 μl of distilled water and 1 μl of Taq polymerase were mixed evenly and placed in a PCR reactor (Applied Biosystems GeneAmp PCR syst...
Embodiment 2
[0037] Example 2 Selection and Cloning of Porcine Immunoglobulin IgG-Fc 4a Fragment Gene
[0038]Fresh pig spleen was taken, and total RNA was extracted by GTC method. Then RT-PCR was used to synthesize cDNA; take 20 μl total RNA, 8 μl 1.25 mM dNTP and 1 μl 3’-end complementary primer, put it on ice after reacting at 70 ° C for 5 minutes, add 1 μl sterilized water, 8 μl 5xAMV RT buffer, 1 μl RNasin and 1 μl AMV reverse transcriptase, and put it at 42°C for 1 hour to synthesize cDNA; then perform PCR reaction on the synthesized cDNA, add 1 μl cDNA, 5 μl 10x PCR reaction solution, 8 μl 1.25mM dNTP, 1 μl5 Forward primer at the ' end, 1 μl reverse primer at the 3' end, 33 μl sterilized water, and 1 μl Taq polymerase were mixed evenly and placed in a PCR reactor (Applied Biosystems GeneAmp PCR system2400). Denaturation followed by 30 cycles of 95°C for 1 minute, 55°C for 30 seconds, and 72°C for 30 seconds, and finally PCR reaction was completed at 72°C for 5 minutes. Wherein, th...
Embodiment 3
[0045] Example 3 Construction of porcine fusion recombinant interferon (P IFN-Fc) DNA sequence
[0046] In this example, the porcine interferon α1 gene (P IFNα1) (SEQ ID No: 1) obtained in Example 1 and the porcine immunoglobulin IgG-Fc 4a fragment gene (P IgG-Fc 4a ) obtained in Example 2 were ) (SEQ ID No: 3) is connected with the DNA sequence (SEQ ID No: 5) of the linker (linker) composed of glycine (Glycine, G) and serine (Serine, S), to construct pig fusion recombinant interferon ( DNA sequence of P IFNα1-Fc 4a) (SEQ ID No: 7).
[0047] First, the porcine interferon α1 gene (P IFNα1) (SEQ ID No: 1) and the porcine immunoglobulin IgG-Fc 4a fragment gene (P IgG-Fc 4a) (SEQ ID No: 3) were respectively amplified by PCR reaction, and The DNA sequence (SEQ ID No: 5) segment of the linker composed of glycine and serine was designed by using PCR primers with the porcine interferon α1 gene (P IFNα1) and the porcine immunoglobulin IgG-Fc 4a fragment gene (P IgG-Fc 4a) Amplify the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com