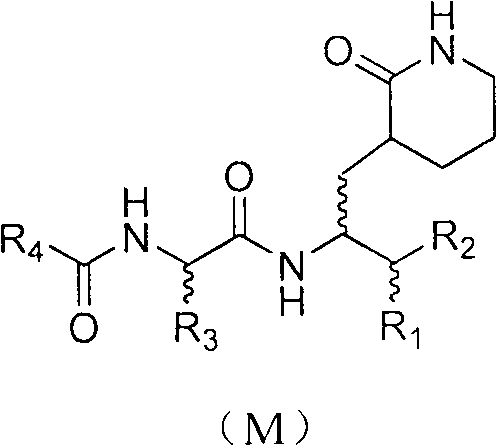
Anti-enterovirus 71 (EV71) caprolactam compounds, and preparation method and application thereof
A technology of caprolactam and EV71, which is applied in the field of formula compounds for the treatment of enterovirus 71 infection, can solve the problem that the virus transcription and replication cannot continue normally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 Preparation of N-Boc-L-(+)-dimethyl glutamate (1-2)
[0111]
[0112] At 0°C, slowly add acetyl chloride (5 mL) dropwise into methanol (100 mL), stir for 5 minutes, then add glutamic acid (10 g, 67.9 mmol), continue stirring and heat to reflux, and keep the reflux temperature for 2 hours . The reaction was stopped, the solvent was removed under reduced pressure, and recrystallized from ether. The obtained oil was dissolved in THF (150mL), TEA (28.5mL, 203.7mmol) was added dropwise at 0°C, kept stirring at 0°C for 5 minutes, and dicarbonate dicarbonate dissolved in HF (30mL) was added dropwise. Tert-butyl ester (17.8 g, 81.5 mmol), stirred to room temperature for 2.5 hours. After the reaction, the solvent was distilled off under reduced pressure, water (200 mL) was added to the residue, extracted from the aqueous phase with DCM (2×200 mL), the combined organic phases were dried over anhydrous sodium sulfate, and then concentrated to obtain the crude produc...
Embodiment 2
[0113] Example 2 Preparation of 2-tert-butoxycarbonylamino-4-cyanoethyl-glutaric acid dimethyl ester (1-3)
[0114]
[0115] At -78°C, lithium bis(trimethylsilyl)amide (78.5mL 1.0M solution in THF, 78.5mmol) was slowly added dropwise to N-Boc-L-(+)-dimethylglutamate ester (1-2) (10 g, 36.4 mmol) in anhydrous THF (200 mL), and the resulting solution was stirred at this temperature for 30 minutes. Then, keeping the temperature constant, bromopropionitrile (3.4 mL) was slowly added dropwise, and the reaction mixture was stirred at -78° C. for 2 hours. After the reaction was complete, glacial acetic acid (5 mL) was added to quench the reaction, and stirred to room temperature. First remove the solvent under reduced pressure, then add water (200mL), extract the aqueous phase with DCM (2×200mL), and dry the combined organic phase with anhydrous sodium sulfate, then concentrate, and the obtained crude product is passed through a flash chromatography column (PE :EA=2:1) to obta...
Embodiment 3
[0116] Example 3 Preparation of 2-tert-butoxycarbonylamino-3-(2-carbonyl-3-piperidinane)-propionic acid methyl ester (1-4)
[0117]
[0118] Add cobalt chloride hydrate (4g, 14.6mmol ), and then slowly added sodium borohydride (6 g, 157.9 mmol) in portions to the obtained pink mixture at 0° C., and stirred at room temperature for 18 hours. The reaction was quenched by adding saturated aqueous ammonium chloride (30 mL), and stirred for 10 minutes. The solid impurities were removed by suction filtration, and the volatile solvent was evaporated under reduced pressure. The aqueous phase was extracted with DCM (3×100 mL), the combined organic phases were dried over anhydrous sodium sulfate, and then concentrated, and the resulting crude product was purified by flash chromatography (EA) to give 2-tert-butoxycarbonylamino-3 -(2-Carbonyl-3-piperidinane)-propionic acid methyl ester (2.9 g, yield 60.7%) was a white foamy solid, TLC: R f =0.4(EA); 1 H-NMR (400MHz, CDCl 3 ): δ6.00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com