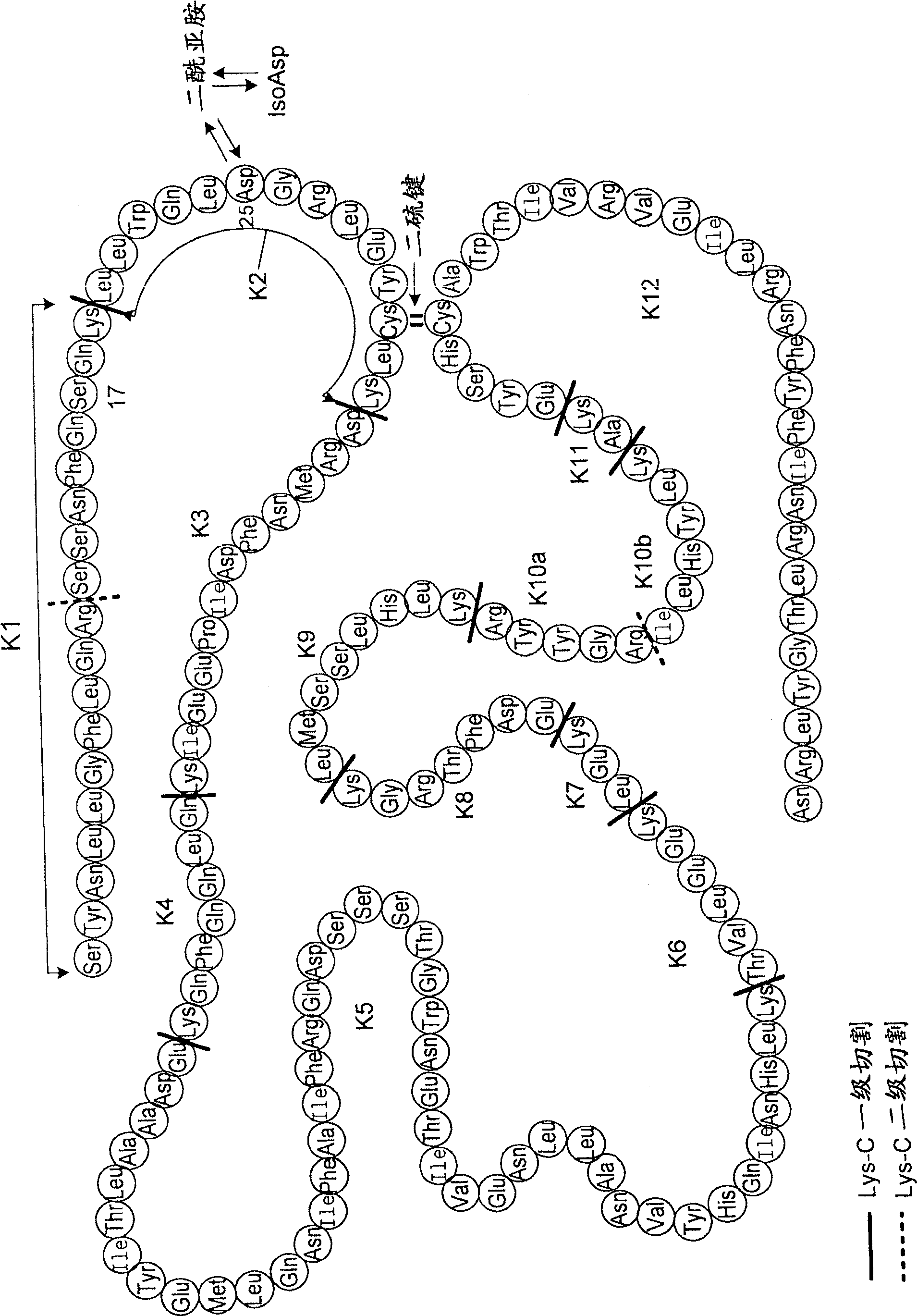Recombinant interferon-beta with enhanced biological activity
A biologically active, interferon-based technology, applied in drug combinations, medical preparations containing active ingredients, allergic diseases, etc., can solve problems such as lack of formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1. Preparation of recombinant Asp25 IFN-β1b.
[0062] A representative composition of the PCR mix used to prepare recombinant deamidated IFN-β1b and a representative PCR protocol are shown in Table 1 and Table 2, respectively.
[0063] Table 1. Composition of the PCR mix
[0064] PCR mix
(51 μL total volume) Template DNA (10
ng / μL) µL
2 µL
5
Forward primer (SEQ
ID NO: 3)(100
ng / μL) 1.25
1.25
Reverse primer (SEQ
ID NO: 4)(100
ng / μL) 1.25
1.25
Buffer 10× 5 5 dNTP mix 1 1 Quick solution 3 3 water 36.5 33.5
Pfu Turbo DNA
polymerase 1 1
[0065] Table 2. Representative PCR Protocols
[0066] PCR protocol duration temperature step 1 1 minute 95℃ step 2 50 seconds 95℃ step 3 50 seconds 60℃ step 4 1 min / kb 68°C, return to step 2, repeat 18 cycles step 5 7 minutes 95℃ ...
Embodiment 2
[0068] Example 2: Increased Potency of Recombinant Asp25 IFN-β1b
[0069] The results of the CPE and Hs294T antiproliferative bioactivity assays are summarized in Tables 3 and 4, respectively. Table 3 shows the CPE activity of Asp 25IFN-β 1b, heat treatment and high pH-treated Asp 25IFN-β 1b, and compared with ( ) compared with the CPE activity of Asn25 IFN-β 1b without HA.
[0070] Table 3: CPE biological activities of IFN-β 1b protein analogs.
[0071] Specific activity (×10 7 IU / mg)
[0072]
[0073]
[0074] Table 4 shows the Hs294T antiproliferative activity of Asp25 IFN-β 1b, compared with HA formulated Compared with the corresponding activity of Asn25 IFN-β1b without HA.
[0075]Table 4. Hs294T antiproliferative activity of IFN-β1b protein analogs
[0076] Specific activity (×10 7 IU / mg)
[0077]
[0078] 2.1. CPE bioassay
[0079] IFN-β induces an antiviral state in mammalian cells in which the replication and cytopathic effects (CPE) of some virus ...
Embodiment 3
[0086] Example 3. Characterization of recombinant Asp25 IFN-β 1b
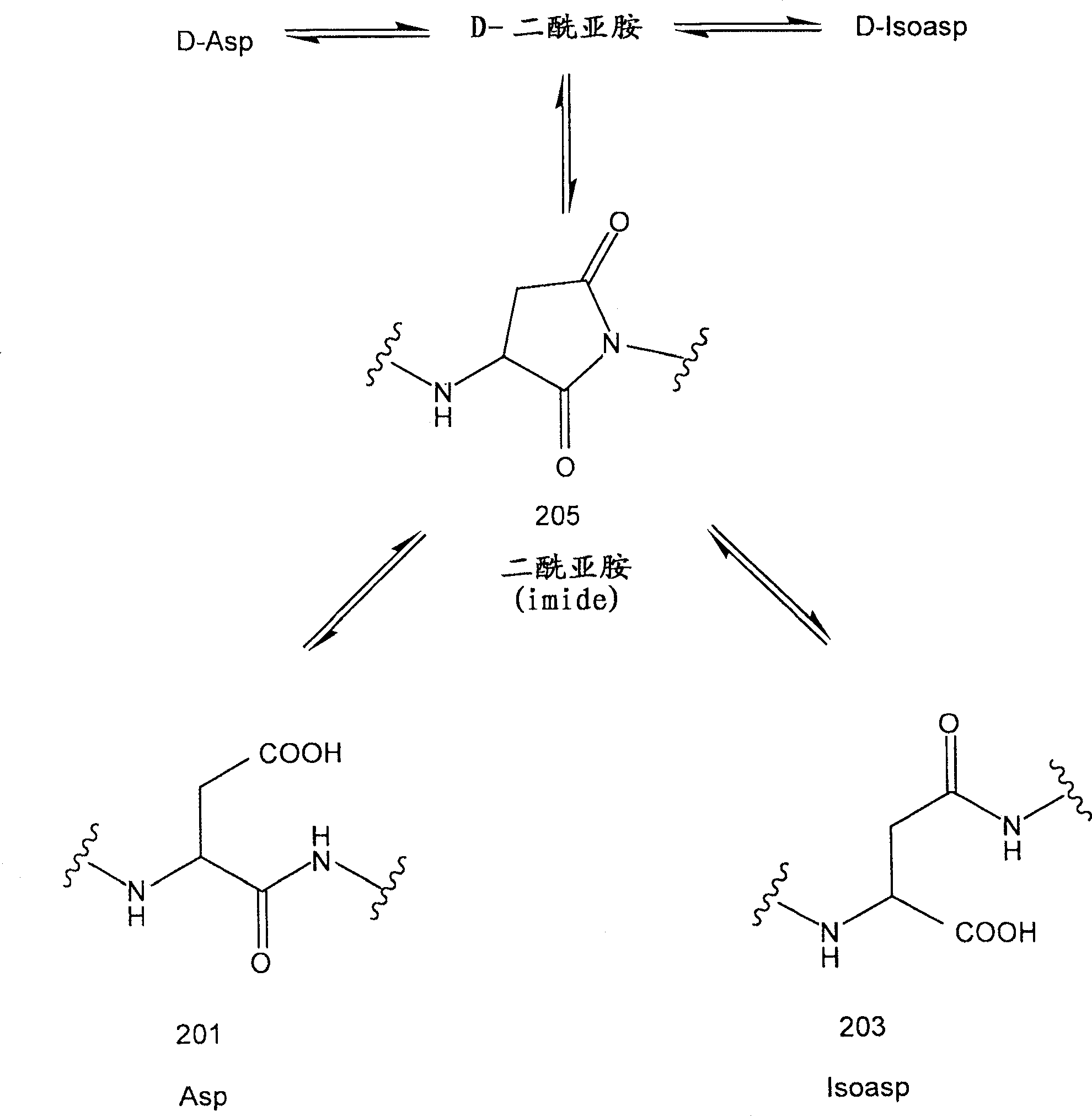
[0087] Recombinant Asp25 IFN-βlb has a recombinant substitution of an aspartic acid residue for an asparagine residue at position 25 (numbering according to natural interferon). It also carries a Cys17Ser mutation. The primary sequence of Asp25 IFN-β1b is shown in image 3 . Aspartic acid at position 25 can be figure 2 The degradation pathway shown converts to succinimide and isoaspartate species. The recombinant IFN-β1b protein analogs of the present invention encompass Asp25 mutant proteins as well as Imide25 and Isoasp25 interferons from Asp25 mutants. The composition of the recombinant IFN-β1b protein analogs of the present invention was investigated in the following assays:
[0088] 3.1 Mapping of reduced Lys-C peptide
[0089] Peptide mapping uses enzyme digestion followed by RP-HPLC to generate fingerprints of proteins. Each peptide fragment isolated by RP-HPLC can be isolated and further charact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com