Canine recombinant interferon-lambda 1, and preparation method and application thereof
A technology for recombining interferon and amino acids, which is applied in the directions of interferon, botanical equipment and methods, biochemical equipment and methods, etc., to achieve high safety and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Optimization of gene sequence encoding canine interferon-λ1 and design and synthesis of primers
[0040] According to the cDNA sequence of canine interferon-λ1 in GenBank (No. AB819731.1), the present invention optimizes the codon and synthesizes the target gene sequence. The codons of the gene encoding canine interferon-λ1 used the most preferred codons of Pichia pastoris. In order to prevent the GC content of the translated mRNA from being too high and the secondary structure of the mRNA from affecting the translation efficiency, the present invention uses a sub-favored code for certain amino acids, provided that the frequency of use of the sub-favored code is very close to that of the most preferred code, and the amino acid sequence The original sequence remains unchanged. In some very special cases, in order to reduce or increase restriction sites, the sequence at certain positions is properly adjusted. Therefore, the present invention optimally designed ...
Embodiment 2
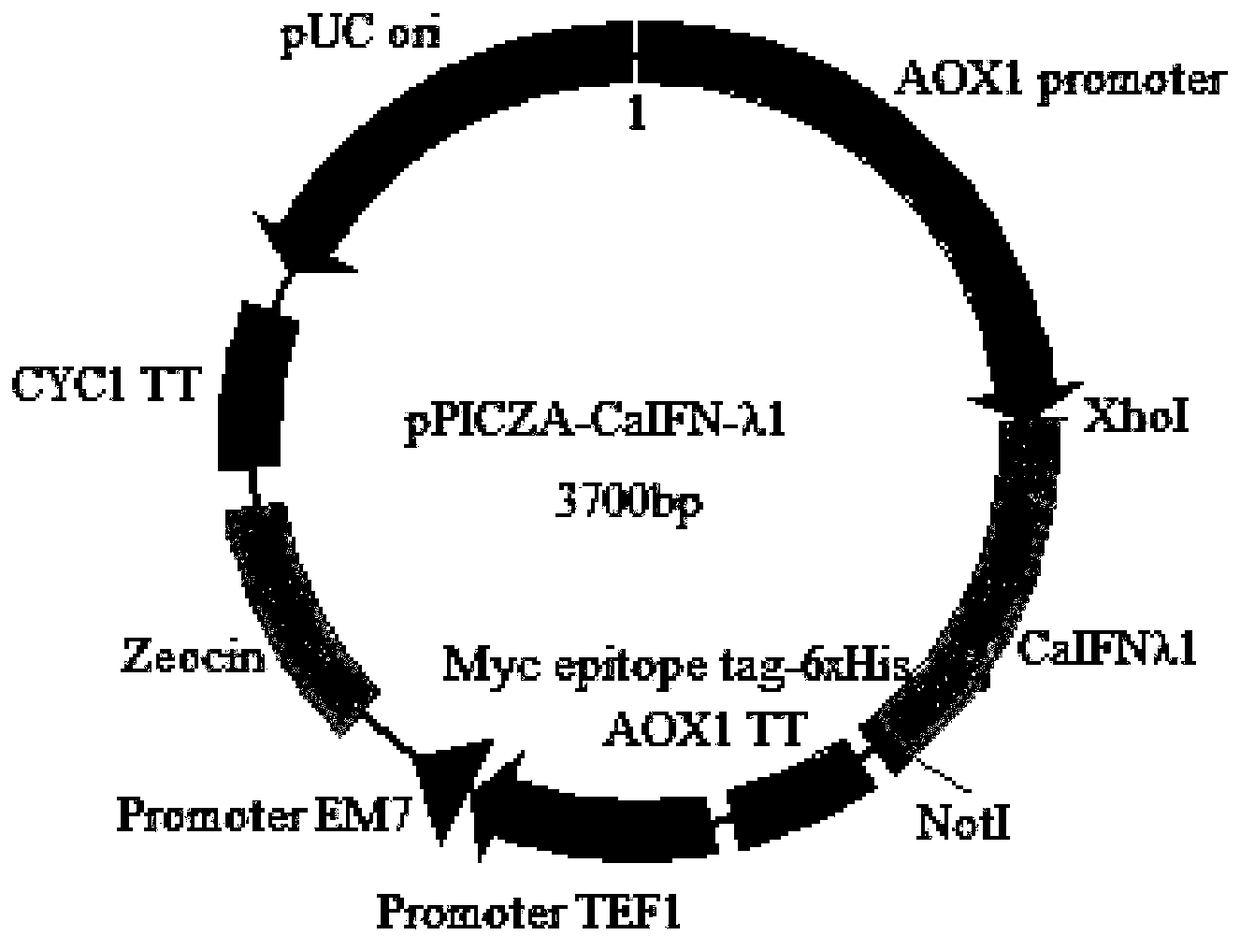
[0041] Example 2 Construction of pPICZαA-CaIFN-λ1 vector
[0042] The CaIFN-λ1 gene fragment and the carrier pPICZαA in Example 1 were double-digested with XhoI and NotI, and the double-digested fragment was recovered. The CaIFN-λ1 gene fragment and the carrier pPICZαA were ligated overnight at 4°C at a molar ratio of 3:1, and transformed into Escherichia coli competent cells DH5α were plated on a low-salt LB plate containing Zeocin (25ug / ml), and cultured at 37°C for 16-24h. Pick a single colony, extract the plasmid, perform PCR identification and send it to Sangon for sequencing. The schematic diagram of the construction of the pPICZαA-CaIFN-λ1 vector is shown in figure 1 .
Embodiment 3
[0043] Example 3 Electroporation Transformation of Yeast Cells and Screening of High Expression Transformants
[0044] Pick colonies with correct PCR and sequencing for expansion culture, select Beyond’s Plasmid Midi Preparation Kit, and carry out plasmid extraction according to the instructions. Prepare Pichia X-33 competent cells according to the Invitrogen PichiaExpression Kit operating instructions. The pPICZαA-CaIFN-λ1 plasmid was linearized using Sac I. See Table 1 for the linearization system.
[0045] Table 1
[0046]
[0047] 37°C overnight. The linearized pPICZαA-CaIFN-λ1 plasmid was subjected to gel electrophoresis, and the size of the result was consistent with the expectation. See the results in figure 2 .
[0048] Mix 200 μl competent cells with 20 μl (10-15 μg) SacI-linearized recombinant plasmid pPICZαA-CaIFN-λ1, inject it into a pre-cooled 0.2 cm electrode cup, place it on ice for several minutes, and place it on the Bio-Rad Gene Pulser electrotransfe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com