Process For the Preparation of Glycosylated Interferon Beta
a glycosylated interferon and beta technology, applied in the field of glycosylated interferon beta preparation, can solve the problems of complex process, increased cost, and many limitations of serum, and achieve the effect of improving the stability of serum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Chinese Hamster Ovary (CHO) Clone Producing IFN-Beta at High Levels
[0164]This Example describes the generation of an interferon beta producing CHO clone.
[0165]The basic procedure, particularly with regard to the preparation of the expression plasmid, is described in Mory et al. (1981).
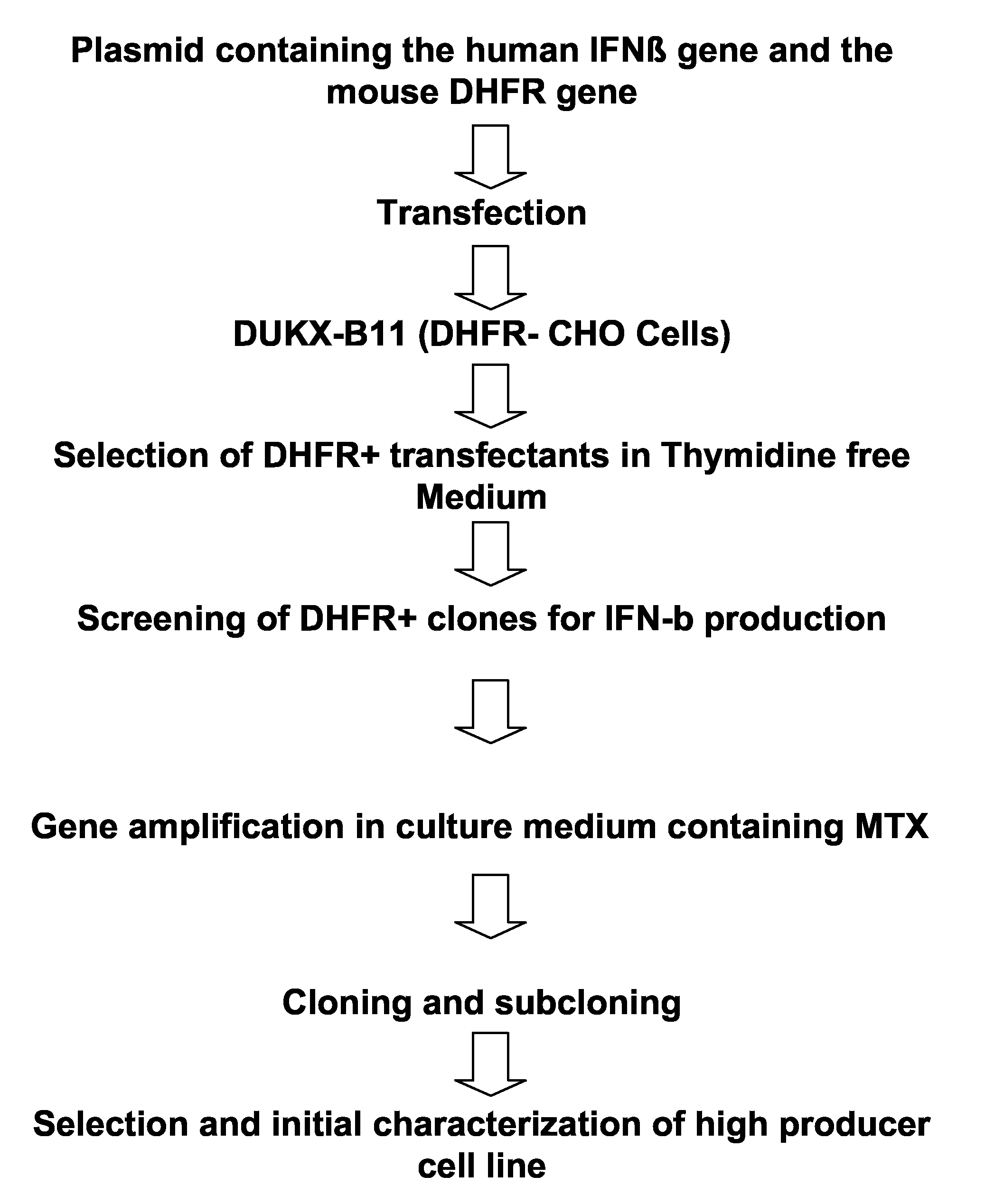
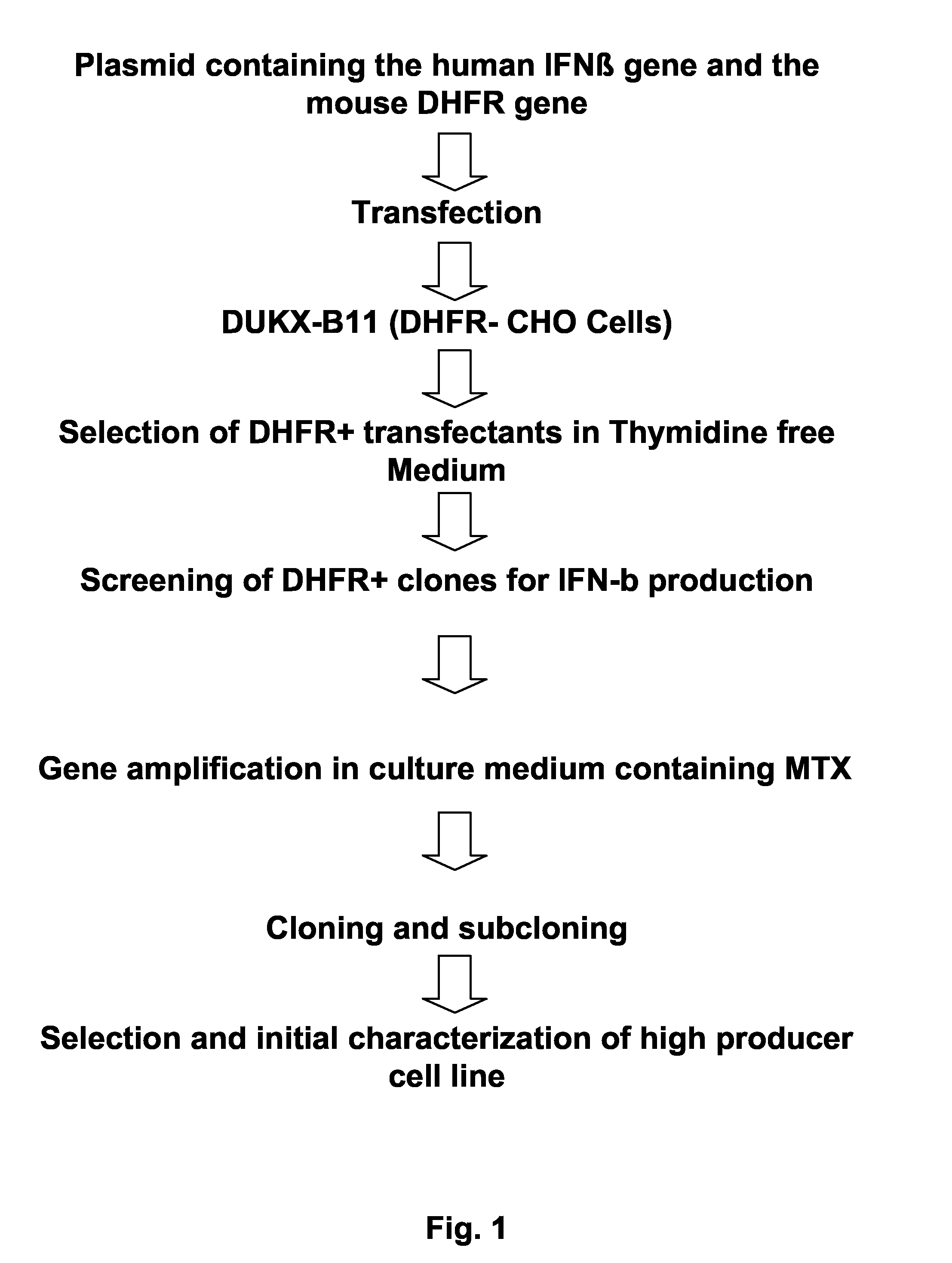
[0166]An overview over the process of generating the clone is depicted in FIG. 1. A DNA fragment comprising the human interferon-β coding region was isolated from a human peripheral blood cell genomic DNA library. A DHFR-deficient CHO cell line was transfected with a recombinant plasmid containing both the human IFN-β coding sequence and the mouse DHFR gene as a selectable and amplifiable marker. After selection in thymidine-free medium, gene amplification with methotrexate (MTX), and cloning, a cell producing IFN-β-1a at high levels was isolated. The cells were subjected to genotypic and phenotypic characterization.
[0167]Construction of the Expression Plasmid Carrying the hIFN-β and t...
example 2
Process for the Production of Interferon Beta
[0247]The overall goal of this experiment was to develop a process for producing IFN beta-1a from the clone described in example 1 under serum-free conditions.
[0248]The serum-free process was developed at 75 L bioreactor scale with internal packed bed Fibra-Cel® carriers.
[0249]The cells were thawed and expanded over 21 days in a commercially available serum-free medium having the following modifications:
TABLE 1Modification of serum-free mediumIngredientsComposition in % (w / w)HEPES 20 mMProline 1 mMPhenol Red 15 mg / LSodium chloride6150 mg / L
[0250]30×109 cells were seeded in the 75 L bioreactor (high seeding).
[0251]The runs were divided into the following phases:[0252]a growth phase I at 37° C. (until working day 2 or working day 4 or when the glucose consumption rate (GCR) was ≧2.0±1.0 g·L−1·d−1)[0253]a growth phase II at 35° C. (until working day 7 or when GCR≧8.0±0.5 g·L−1·d−1)[0254]a production phase at 33° C.
[0255]This temperature stra...
example 3
Purification of the Interferon Protein Product
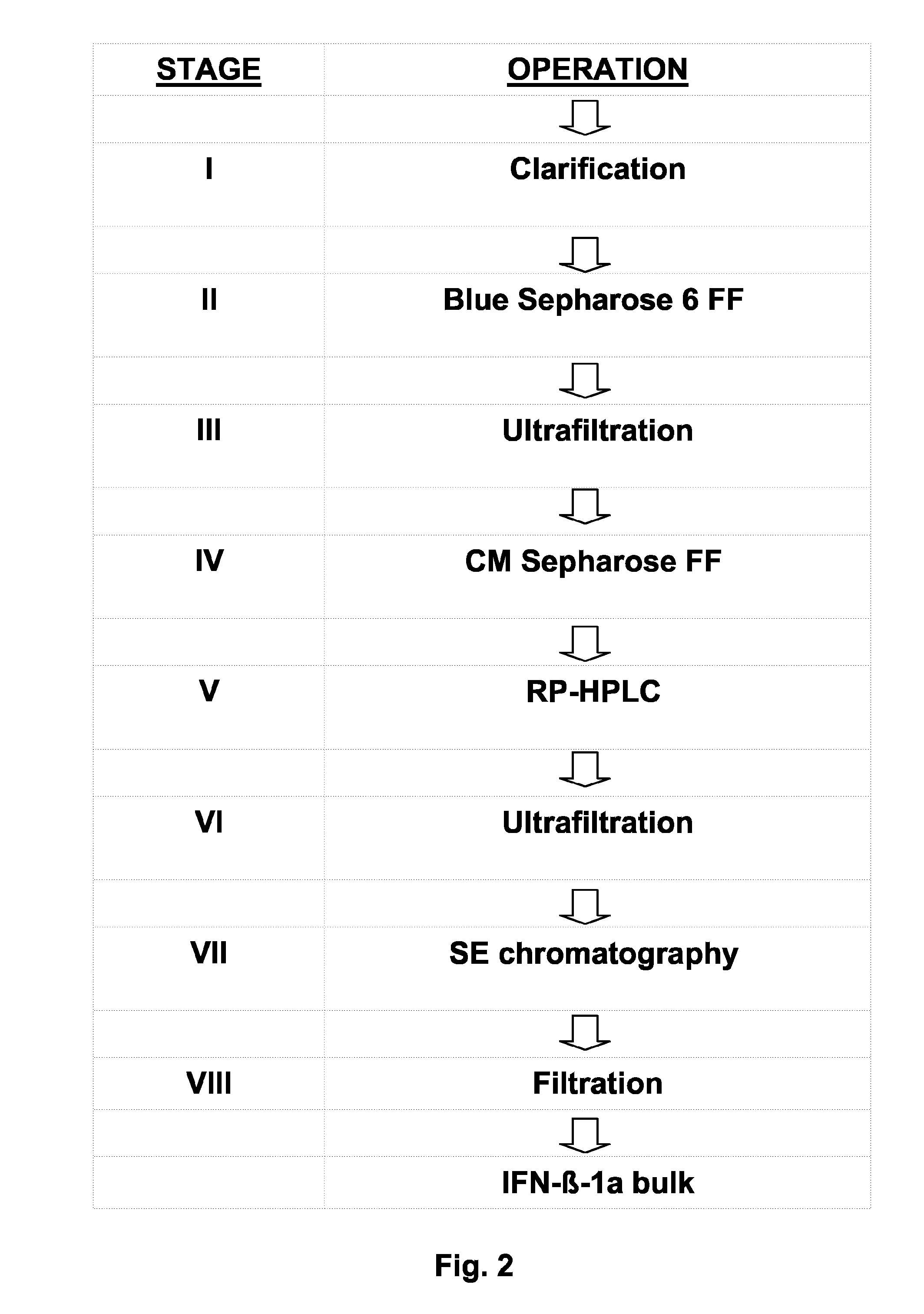
[0260]The purification process of the IFN-β-1a from cell culture supernatant included four chromatographic and four filtration stages, as shown in FIG. 2. The purification stages were performed in the following order:[0261]Stage I: Harvest clarification by filtration[0262]Stage II: Affinity chromatography on a Blue Sepharose 6 fast flow (6 FF) column;[0263]Stage III: Ultrafiltration[0264]Stage IV: Cation exchange chromatography using preferably a CM Sepharose FF column;[0265]Stage V: Hydrophobic chromatography RP-HPLC;[0266]Stage VI: Ultrafiltration and dialysis;[0267]Stage VII: Size Exclusion (SE) chromatography;[0268]Stage VIII: Microfiltration.
[0269]The purification of the active ingredient started with dye affinity chromatography on Blue Sepharose 6 FF (BS 6 FF), which was the major purification stage. The amount of host cell derived proteins as well as DNA from ruptured CHO cells was reduced by several orders of magnitude and theref...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com