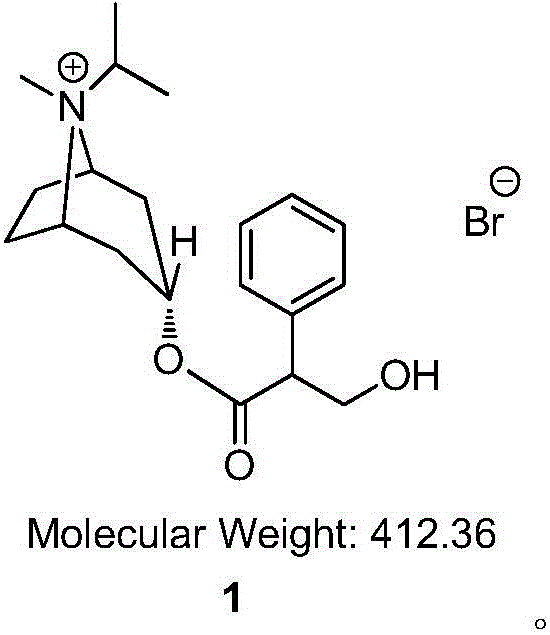
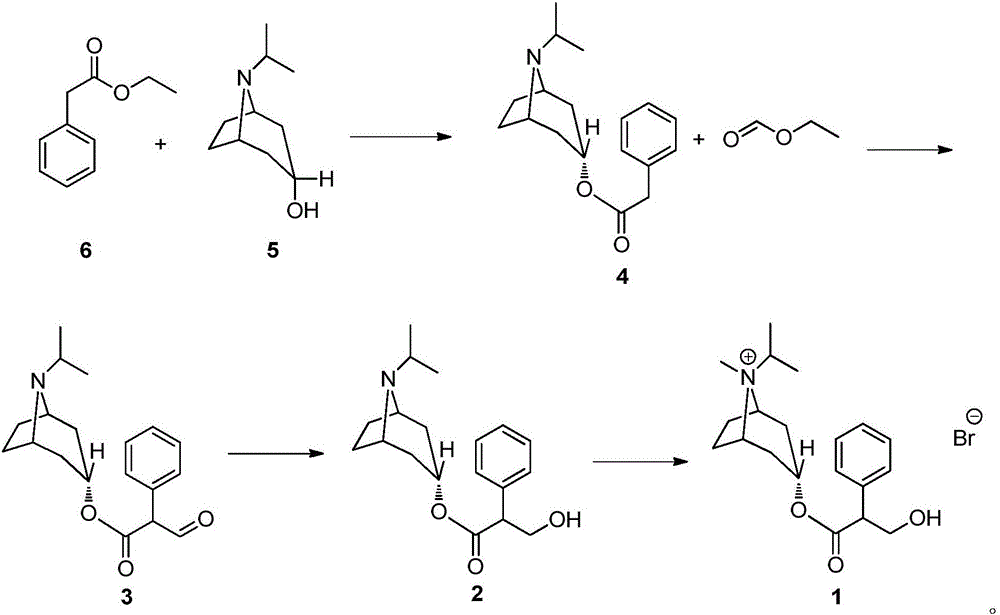
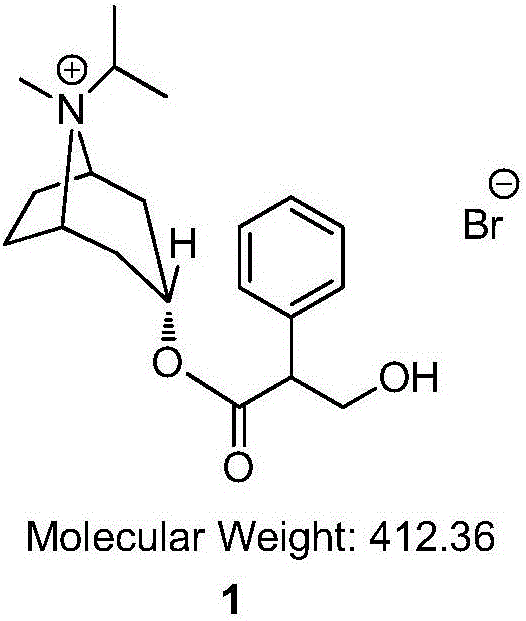
Preparation method of ipratropium bromide
A technology of ipratropium bromide and ipropyltropine alcohol, applied in the direction of organic chemistry, can solve the problems of poor reproducibility of the preparation process, unfavorable large-scale preparation, and great harm to experimenters, so as to avoid flammable and explosive and the use of toxic reagents, reducing workers' labor protection requirements, and reducing the effect of workshop equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of Intermediate 4
[0040] In the 250mL reaction flask, add isopropyltropine alcohol (5g), ethyl phenylacetate (7.3g), tetrahydrofuran (30mL) successively, stir to dissolve clear, cool to 0 ℃, add sodium hydride (0.78g), T= 10 ~ 20 ℃ reaction 3h. After the TLC detection reaction was complete, dilute hydrochloric acid (1.5mol / L, 30mL) was added to the system, dichloromethane (60mL) was extracted, and the organic layer was washed with 5% aqueous sodium carbonate solution (30ml), washed with water (30ml), dried, and reduced Concentrated under reduced pressure to obtain intermediate 4 (5.6 g, yield 66.0%) with a purity of 95.90% (HPLC normalization method). ESI-MS (C 18 h 25 NO 2 ,m / z) measured value (calculated value): 287.12 (287.40) [M-H] - .
[0041] (2) Synthesis of intermediate 3
[0042] Add intermediate 3 (5.0 g), methyl formate (50 mL), and potassium (1.02 g) to the reaction flask in sequence, and react at T=10-20° C. for 6 h. After the reacti...
Embodiment 2
[0048] (1) Synthesis of Intermediate 4
[0049] Add isopropyltropine alcohol (10g), ethyl phenylacetate (14.6g), and benzene (60mL) successively into a 250mL reaction flask, stir to dissolve, cool to -10°C, add sodium ethoxide (0.78g), T = 0 ~ 10 ℃ reaction 5h. After the TLC detection reaction was complete, dilute hydrochloric acid (0.5mol / L, 60mL) was added to the system, dichloromethane (60mL) was extracted, and the organic layer was washed with 5% potassium hydroxide aqueous solution (60ml), washed with water (60ml), and dried. Concentration under reduced pressure gave intermediate 4 (13.7 g, yield 80.7%) with a purity of 96.31% (HPLC normalization method). ESI-MS (C 18 h 25 NO 2 ,m / z) measured value (calculated value): 287.51 (287.40) [M-H] - .
[0050] (2) Synthesis of intermediate 3
[0051] Intermediate 3 (13.0 g), ethyl formate (130 mL), and lithium (0.47 g) were sequentially added to the reaction flask, and reacted at T=15-25° C. for 4 h. After the reaction wa...
Embodiment 3
[0057] (1) Synthesis of Intermediate 4
[0058] In the 250mL reaction flask, add isopropyltropine alcohol (5g), ethyl phenylacetate (7.3g), toluene (50mL) successively, stir to dissolve clear, cool to 0 ℃, add sodium methylate (1.8g), T= 20 ~ 30 ℃ reaction 2h. After TLC detection reaction is complete, add dilute hydrochloric acid (2mol / L, 50mL) in the system, extract, add dichloromethane (50mL) in the aqueous phase, extract, organic layer 5% sodium hydroxide aqueous solution (30ml) washes, washes with water (30ml), dried, and concentrated under reduced pressure to obtain intermediate 4 (8.0g, yield 94.4%) with a purity of 97.60% (HPLC normalization method). ESI-MS (C 18 h 25 NO 2 ,m / z) measured value (calculated value): 287.30 (287.40) [M-H] - .
[0059] (2) Synthesis of Intermediate 3
[0060] Intermediate 3 (5.0 g), ethyl formate (25 mL), and sodium (0.60 g) were sequentially added to the reaction flask, and reacted at T=20-30° C. for 4 h. After the reaction was dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com