A kind of detection method of impurity a in different third detropammonium bromide
A technology of ipratropium bromide and detection method is applied in the detection field of impurity A in ipratropium bromide, and achieves the effects of short detection time, realization of baseline separation and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Determination of Impurity A in Ipratropium Bromide by Ion Chromatography
[0054] Instrument and chromatographic conditions:
[0055] Thermo Fisher DIONEX ICS-2100 ion chromatograph;
[0056] Detector: conductivity detector;
[0057] Suppressor: Cationic Suppressor CSRS 300, 4mm;
[0058] Chromatographic column: the analytical column is IonPac CS17 4×250mm, 3.5μm; the pre-column is IonPac CG17 4×50mm;
[0059] Column temperature 35°C;
[0060] Carry out isocratic elution with 5mmol / L methanesulfonic acid aqueous solution-acetonitrile (60:40), flow rate 1.0ml / min; injection volume 500μl.
[0061] Specific detection steps:
[0062] Step 1. Preparation of the test solution: Accurately weigh 50 mg of ipratropium bromide raw material and place it in a 10ml volumetric flask, dilute to the mark with ultrapure water solvent, shake well, and you get it.
[0063] Step 2. Preparation of reference substance solution: Accurately weigh an appropriate amount of ipra...
Embodiment 2
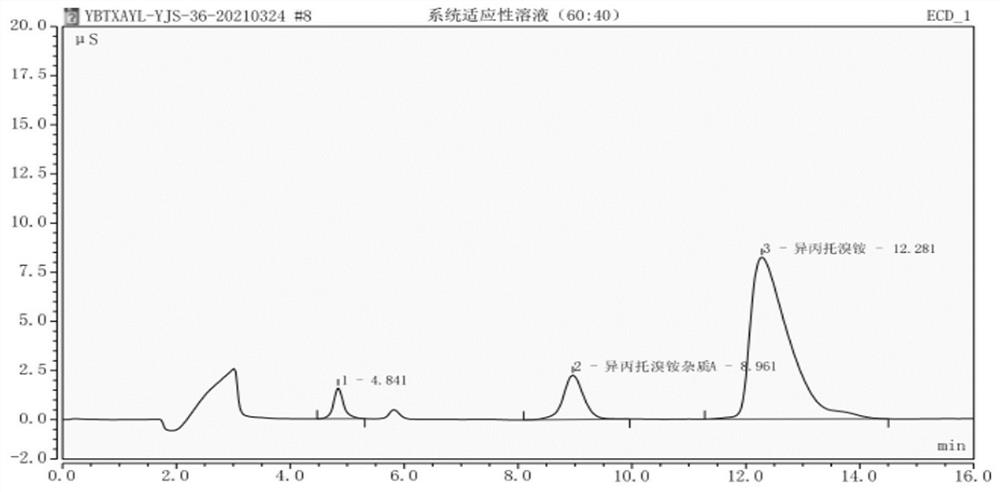
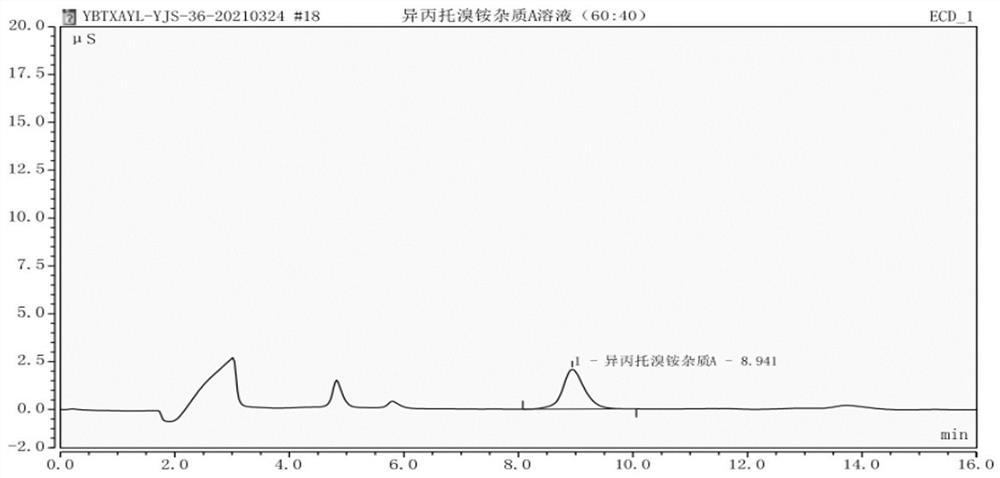
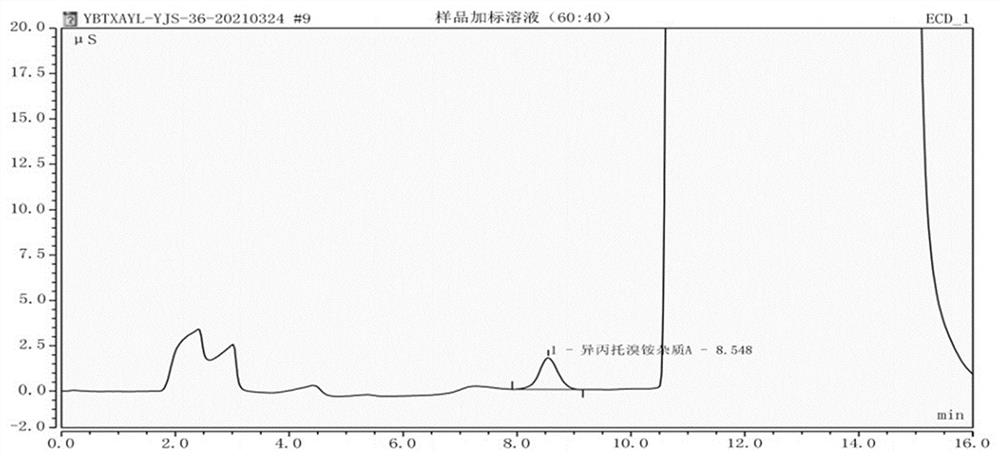
[0067] Example 2: The specificity test of detection method of the present invention
[0068] The specificity test was carried out under the same chromatographic conditions as in Example 1.
[0069] Specific test steps:
[0070] Step 1. Preparation of blank solution, ie ultrapure water.
[0071] Step 2. The preparation of the system suitability solution is consistent with the system suitability solution in Example 1.
[0072] Step 3. The preparation of the reference substance solution is consistent with the reference substance solution in Example 1.
[0073] Step 4. Sample spiked solution: Precisely weigh 50 mg of the sample and place it in a 10ml volumetric flask, dissolve it with the reference solution to make up the volume, and shake well to get it.
[0074] Step 5. Preparation of positioning solution: take an appropriate amount of sodium chloride and dissolve and dilute it with ultrapure water to obtain a sodium ion positioning solution; take an appropriate amount of p...
Embodiment 3
[0078] Example 3: The linear range test of detection method of the present invention
[0079] The same method as in Example 1 was used to carry out the linear range test.
[0080] The results showed that the impurity A of ipratropium bromide was in the concentration range of L-200% (0.5-10μg / ml), and the concentration was taken as the abscissa, and the peak area was taken as the ordinate to carry out linear regression, and the linear correlation coefficient r was 0.9993. It shows that the method of the present invention has a good linear relationship in the concentration range of L-200% (0.5-10 μg / ml), and is suitable for the quantitative detection of trace ipratropium bromide impurity A.
[0081] The linear range test method in this embodiment belongs to the prior art.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com