Salbutamol sulfate solution for inhalation and preparation method thereof
A technology of salbutamol sulfate and solution, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as poor stability, improve tolerance, improve stability, The effect of ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
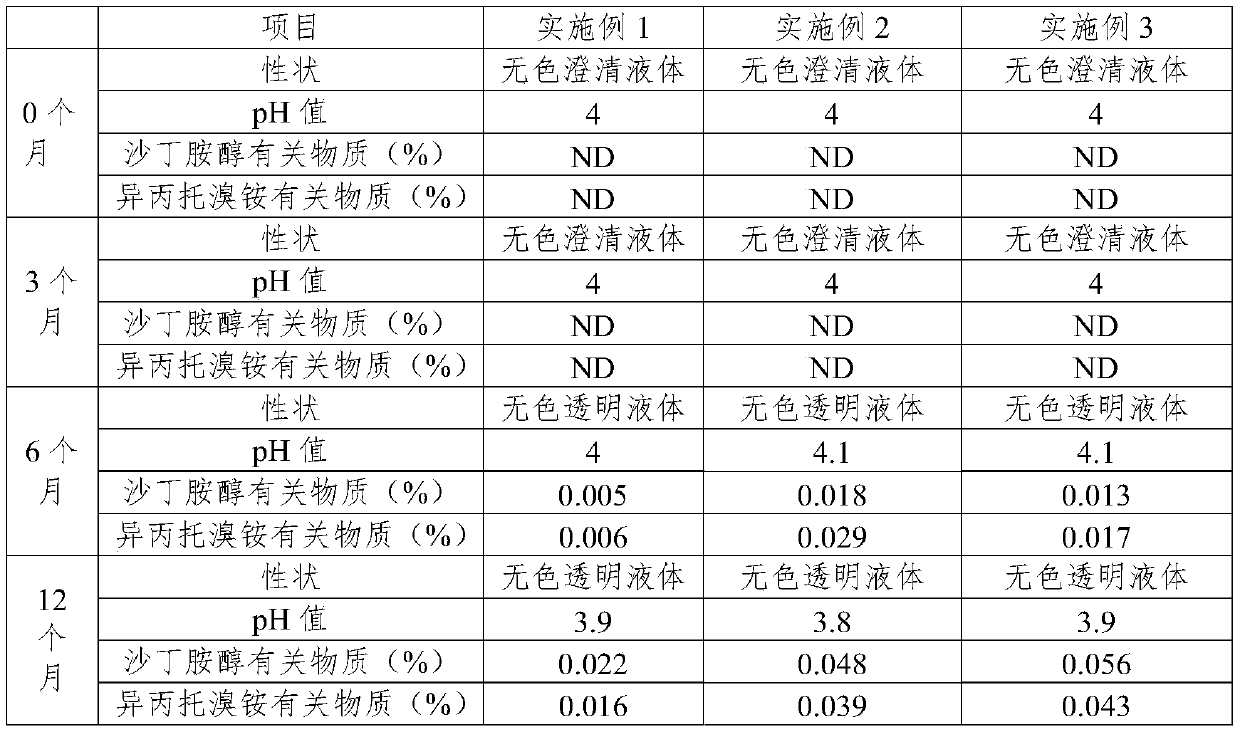
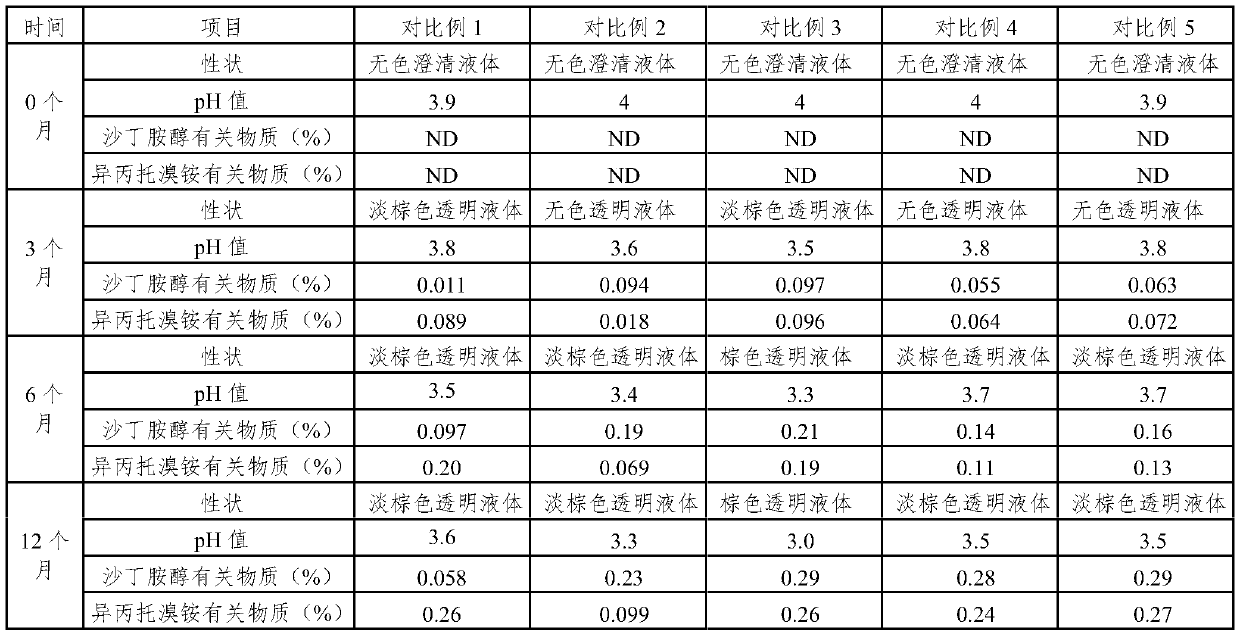
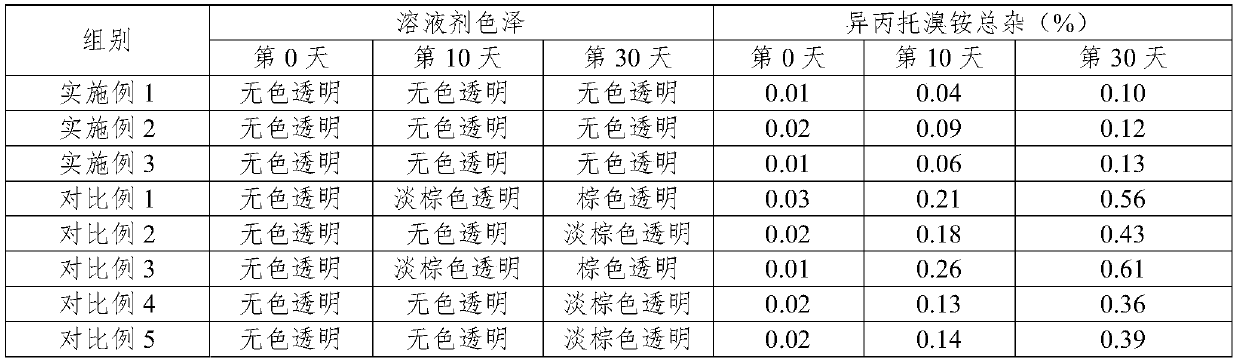
Embodiment 1
[0029] prescription:
[0030] Salbutamol Sulfate 1.0g
[0031] Ipratropium Bromide 0.2g
[0032] Sodium Hyaluronate 0.4g
[0033] Carboxymethyl Chitooligosaccharide 0.2g
[0035] The pH regulator is 8% hydrochloric acid solution.
[0036] Makes 1000ml.
[0037] Preparation Process:
[0038] S1. Weighing and dispensing: Prepare raw and auxiliary materials according to the prescription amount in the D-level clean area, and transfer them to the C-level clean area after exposure and sterilization;
[0039] S2. Dosing: In the C-level clean area, add albuterol sulfate, ipratropium bromide, isotonic regulator, sodium hyaluronate, and carboxymethyl chitosan oligosaccharide to the liquid mixing tank in sequence, and the liquid mixing tank is added in advance Water for injection, fully stirred and mixed after filling with nitrogen;
[0040] S3, adjust the pH: adjust the pH to 4 with a pH regulator;
[0041] S4. Filtration: pass the solution through...
Embodiment 2
[0044] prescription:
[0045] Salbutamol Sulfate 1.0g
[0046] Ipratropium Bromide 0.3g
[0047] Sodium Hyaluronate 0.5g
[0048] Carboxymethyl Chitooligosaccharide 0.25g
[0049] Sodium chloride 7.8g
[0050] The pH regulator is citric acid-sodium citrate solution.
[0051] Makes 1000ml.
[0052] The preparation method is the same as in Example 1.
Embodiment 3
[0054] prescription:
[0055] Salbutamol Sulfate 1.0g
[0056] Ipratropium Bromide 0.125g
[0057] Sodium Hyaluronate 0.3g
[0058] Carboxymethyl Chitooligosaccharide 0.15g
[0059] Sodium chloride 8.2g
[0060] The pH regulator is 10% hydrochloric acid solution.
[0061] Makes 1000ml.
[0062] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com