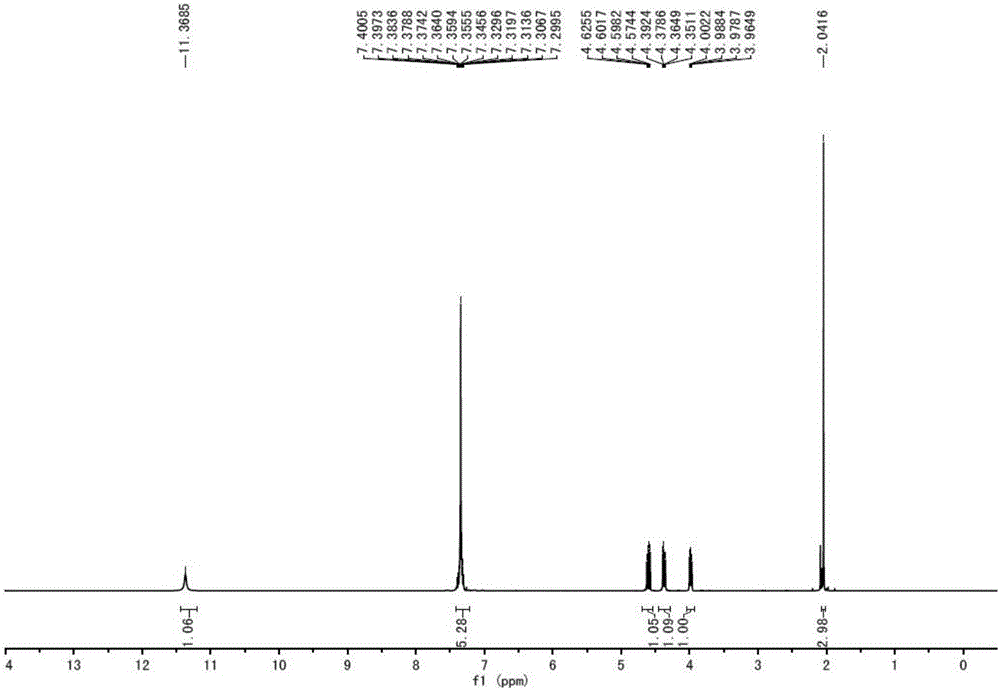
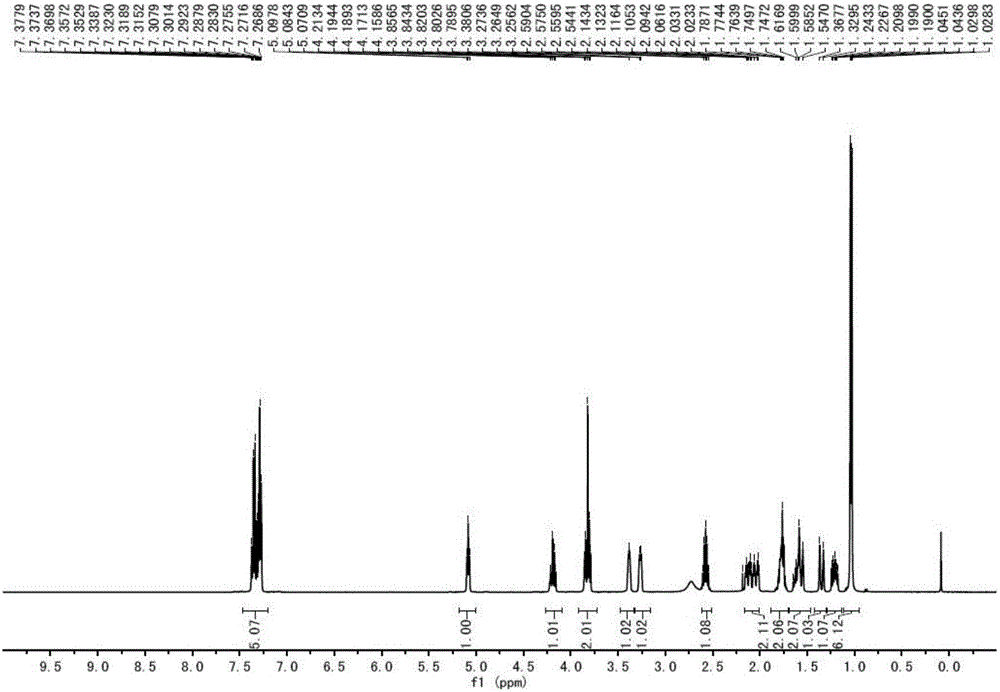
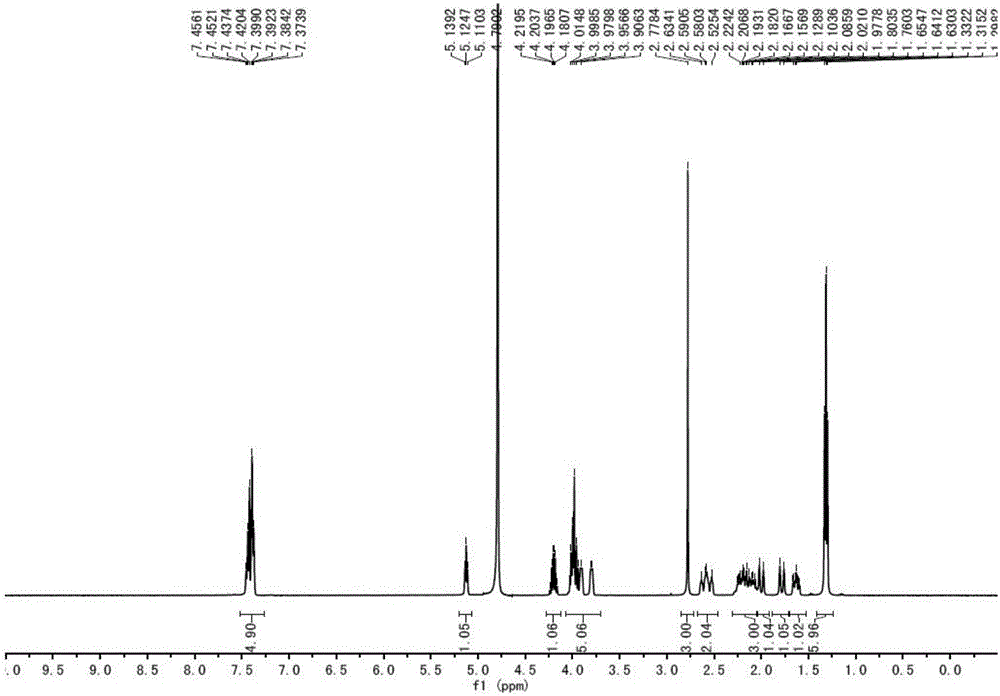
Synthesis method of ipratropium bromide
A kind of technology of ipratropium bromide, synthetic method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The present embodiment provides a kind of synthetic method of ipratropium bromide, specifically comprises the steps:
[0042] (1) Preparation of compound 1
[0043] Add 50 grams (0.3mol) of tropic acid and 59 grams (0.75mol) of acetyl chloride in the reaction bottle, insulate at 20~30 ℃ for 2 hours, and the reaction solution is concentrated to dryness under reduced pressure to obtain 63 grams of colorless oily product, namely It is compound 1, which can be directly used in the next reaction. The HPLC purity of Compound 1 was detected to be 95.2%.
[0044] (2) Preparation of Compound 2
[0045] Add 60 grams (0.29mol) of compound 1 and 180 milliliters of thionyl chloride into the reaction flask, stir and react at 50-60°C for 2 hours, concentrate the reaction solution to dryness under reduced pressure, add 100 milliliters of toluene to the concentrate, and then reduce Concentrate to dryness under reduced pressure to remove residual thionyl chloride to obtain 65 g of a c...
Embodiment 2
[0054] The present embodiment provides a kind of synthetic method of ipratropium bromide, specifically comprises the steps:
[0055] (1) Preparation of compound 1
[0056] Add 20 grams (0.12mol) of tropic acid and 9.5 grams (0.12mol) of acetyl chloride in the reaction bottle, insulate at 40~50 ℃ and react for 2 hours, and the reaction solution is concentrated to dryness under reduced pressure to obtain 25.2 grams of colorless oily product, i.e. It is compound 1, which can be directly used in the next reaction. After detection, the HPLC purity of the obtained compound 1 was 92.8%.
[0057] (2) Preparation of Compound 2
[0058] Add 25 grams (0.12mol) of compound 1 and 75 milliliters of oxalyl chloride into the reaction flask, stir and react at 20-40 °C for 2 hours, concentrate the reaction solution to dryness under reduced pressure, add 50 milliliters of toluene to the concentrate, and then concentrate under reduced pressure To dryness, remove the residual oxalyl chloride to...
Embodiment 3
[0067] The present embodiment provides a kind of synthetic method of ipratropium bromide, specifically comprises the steps:
[0068] (1) Preparation of compound 1
[0069] Add 20 grams (0.12mol) of tropic acid and 47.2 grams (0.6mol) of acetyl chloride in the reaction bottle, insulate at 20~30 ℃ and react for 2 hours, and the reaction solution is concentrated to dryness under reduced pressure to obtain 25.1 grams of colorless oily product, i.e. It is compound 1, which can be directly used in the next reaction. After detection, the HPLC purity of the obtained compound 1 was 93.2%.
[0070] (2) Preparation of compound 2
[0071] Add 25 grams (0.12mol) of compound 1 and 75 milliliters of phosphorus trichloride to the reaction flask, stir and react at 60-80°C for 2 hours, concentrate the reaction solution to dryness under reduced pressure, add 50 milliliters of toluene to the concentrate, and then reduce Concentrate to dryness under reduced pressure to remove residual phosphoru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com