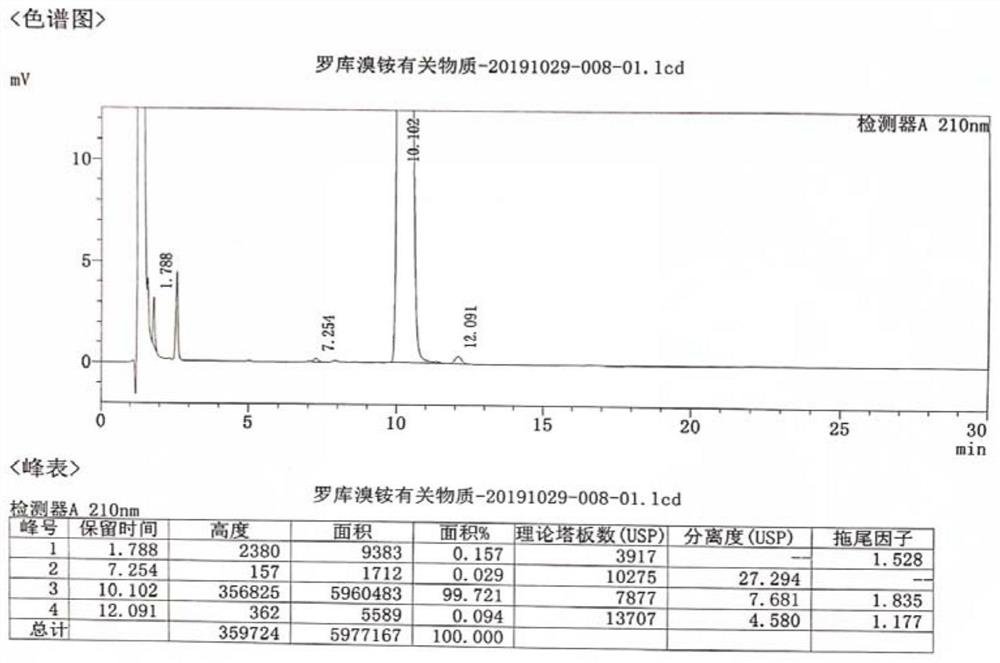
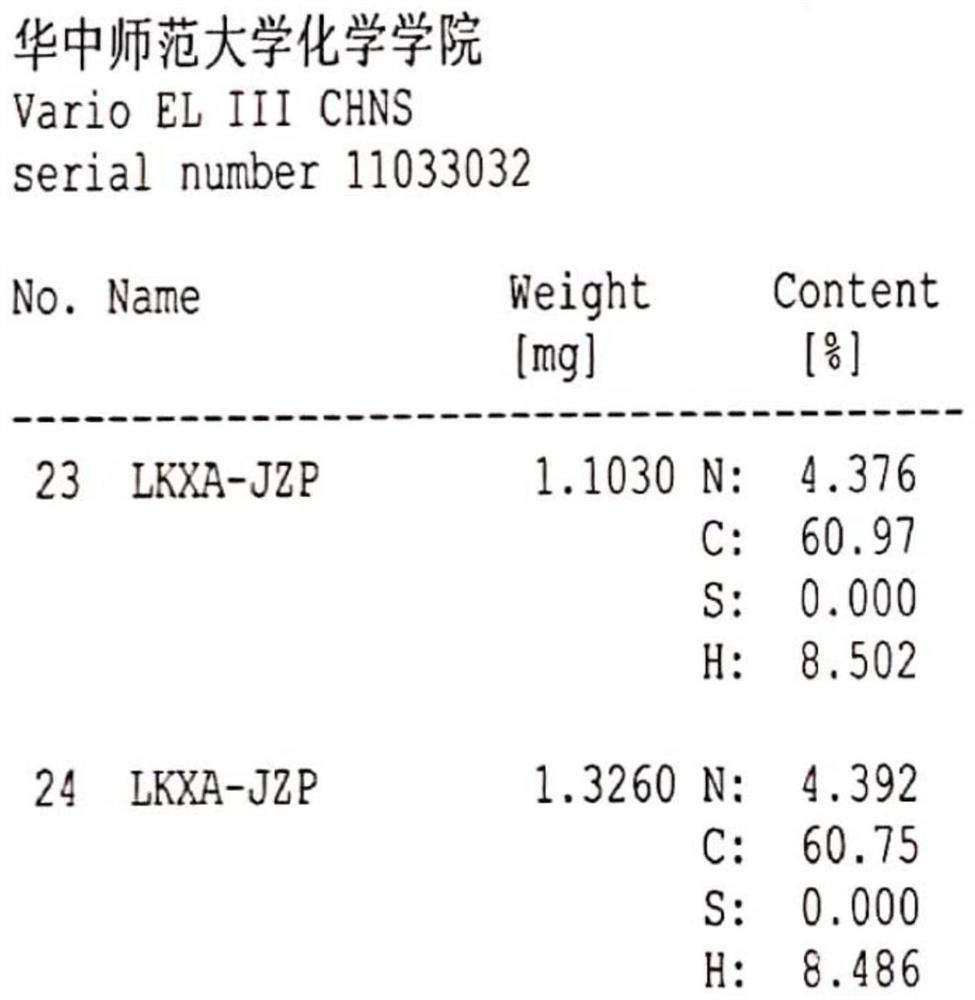
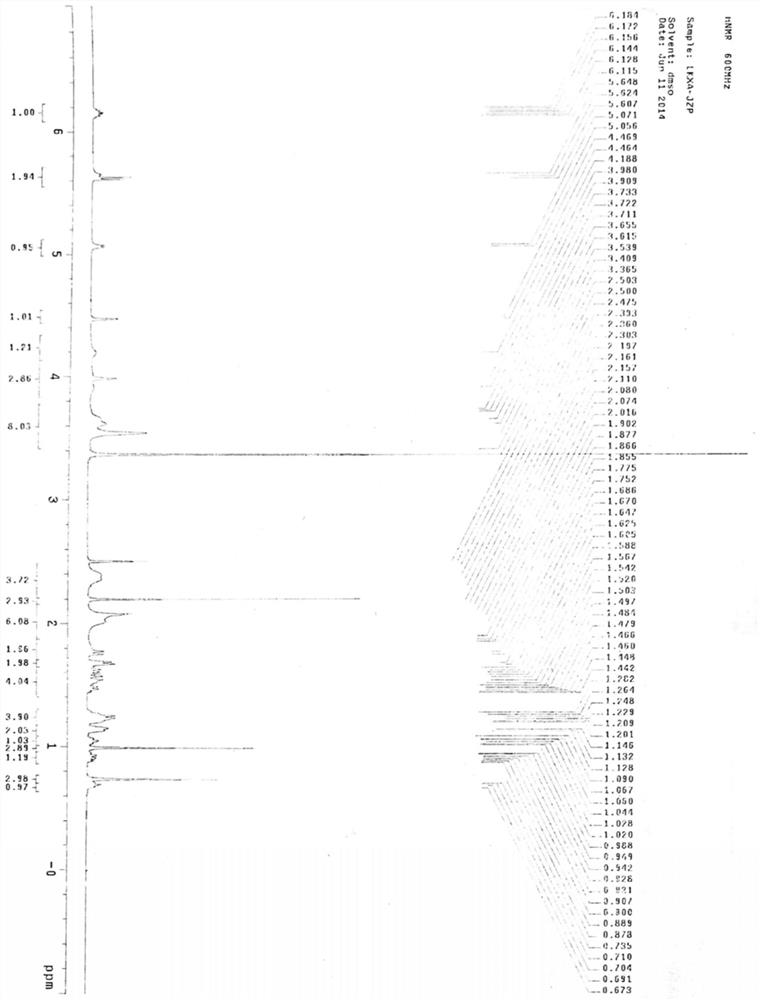
A kind of preparation method of rocuronium bromide
A technology for rocuronium bromide and a compound, which is applied in the field of substance synthesis, can solve the problems of low yield, excessive residual impurities and solvent, easily excessive hydrolyzed impurities, etc., achieves good effect of removing solvent and residual, simplifies the difficulty of subsequent processing, The effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 of the present invention provides a kind of preparation method of rocuronium bromide, comprises the steps:
[0038](1) Preparation of Compound III
[0039] Pump 72kg of dichloromethane into the reaction kettle at 25°C-30°C, start stirring, add 1.6kg of starting materials, stir and disperse; add 0.63kg of acetyl chloride dropwise, after dropping, keep the temperature of the material liquid at 25°C-30°C and stir React for 5 to 8 hours, the reaction solution is not processed, directly dropwise add 4.1kg of 10% hydrochloric acid solution to the reaction solution, after the drop is complete, keep the reaction at 25°C to 30°C for 20 hours, let stand and separate, remove 46.7kg of the organic layer, Add about 20.6 kg of 10% sodium carbonate aqueous solution to the remaining reaction solution to adjust pH=7 to 9, separate the organic phase, add 3.8 kg of silica gel to the organic phase, stir and absorb for 10 min, filter under reduced pressure, and then use 32.3 kg...
Embodiment 2
[0044] In this embodiment, except step 3 is changed to "the mass ratio of dichloromethane to methyl tert-butyl ether-ethyl acetate in the dichloromethane-methyl tert-butyl ether-ethyl acetate system is 8:20:20" , all the other are with embodiment 1.
Embodiment 3
[0046] In this embodiment, except that step 3 is changed to "the mass ratio of dichloromethane to methyl tert-butyl ether-ethyl acetate in the dichloromethane-methyl tert-butyl ether-ethyl acetate system is 3:25:25" , all the other are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com