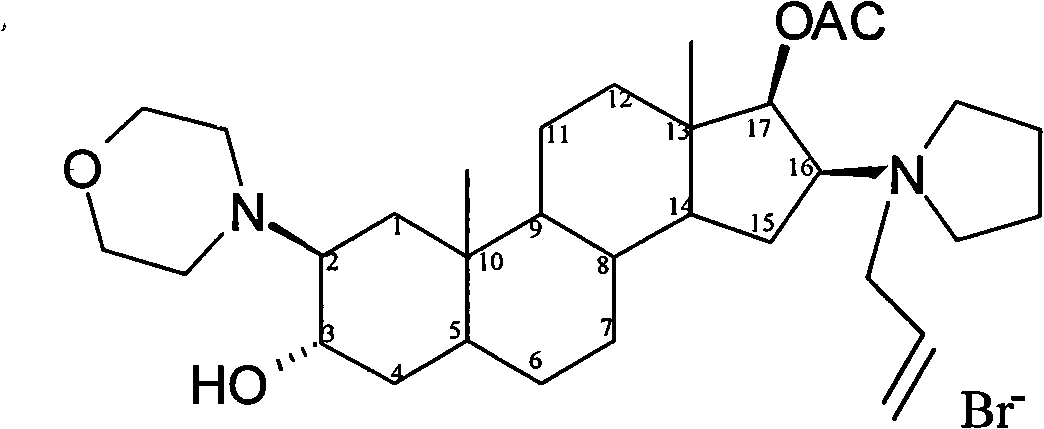
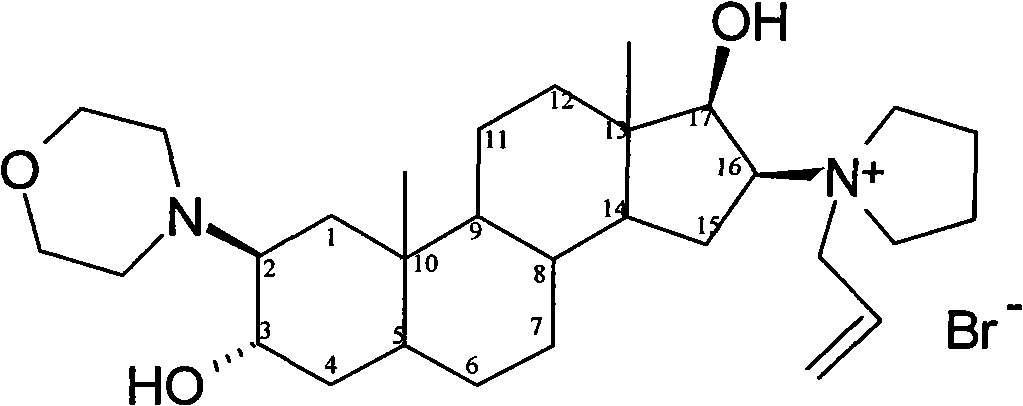
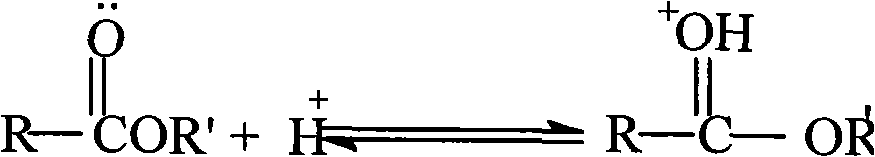
Stable rocuronium bromide composition for injection
A rocuronium bromide, stable technology, applied in the field of stable rocuronium bromide injection composition and its preparation, can solve the problems of increased impurity C content, high production cost, low sterility assurance level, etc., and achieve the reduction of impurities The effect of C
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The comparison of the stabilizing effect of different metal chelating agents of embodiment 1
[0032] The formula of rocuronium bromide injection is shown in Table 1:
[0033] Table 1 Rocuronium bromide injection formula table
[0034] prescription number
1
2
3
4
5
6
7
50g
50g
50g
50g
50g
50g
50g
10g
10g
10g
10g
10g
10g
10g
EDTA-2Na
15g
-
-
-
-
-
-
EDTA-2Na-Ca
-
15g
-
-
-
-
-
Sodium cyclohexanediamine tetraacetate
-
-
15g
-
-
-
-
Dimercaptopropanol
-
-
-
15g
-
-
-
-
-
-
-
15g
-
-
Sodium Dithiopropanesulfonate
-
- ...
Embodiment 2
[0039] The screening of embodiment 2EDTA-2Na or EDTA-2Na-Ca concentration range
[0040] The formula of rocuronium bromide injection is shown in Table 3:
[0041] Table 3 Rocuronium Bromide Injection Formula Table
[0042] prescription number
[0043] Preparation process: Weigh the prescribed amount of acetic acid according to the prescription in the above table, add more than 80% of the prescribed amount of water for injection to dissolve, add sodium hydroxide to adjust the pH value to 4.0, add other excipients and drugs in turn, stir to dissolve, sodium hydroxide or acetic acid Adjust the pH value to 4.0, add 0.1% (weight / volume) of the solution volume, stir and adsorb pyrogen with activated carbon, filter for decarburization, add water for injection to the full amount, divide into ampoules according to the unit dose of 5ml, seal, and keep at 115°C , autoclave for 30 minutes to get the finished product.
Embodiment 3
[0046] The formula of rocuronium bromide injection is shown in Table 4:
[0047] Table 4 Rocuronium Bromide Injection Formula Table
[0048] prescription number
[0049] Preparation process: take the citric acid (or acetic acid) of the prescription amount according to the prescription in the above table, add more than 80% of the prescription amount to dissolve in water for injection, add sodium hydroxide to adjust the pH value to within the range of 4.0, add other auxiliary materials and drugs in turn, Stir to dissolve, adjust the pH value to 4.0 with sodium hydroxide or acetic acid (citric acid), add 0.1% (weight / volume) activated carbon to the solution volume, stir to absorb pyrogen, filter for decarburization, and add water for injection to the full amount, according to the unit Dosage of 5ml is divided into ampoules, sealed, and sterilized at 115°C for 30 minutes by autoclaving to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com