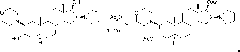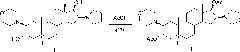Method for preparing rocuronium bromide midbody compound crystal
A technology of rocuronium bromide and compounds, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of large solution volume, high cost, complicated operation, etc., and achieve the effects of convenient and effective method, shortened reaction time, and improved product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
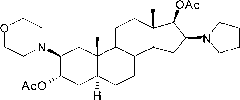
[0038] Preparation of compound 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androstane-3α, 17β-diol-diacetate crystals:
[0039] In the reaction flask, add 120ml of dichloromethane, compound 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androstane-3α, 30g of 17β-diol (II), and stir , cooled to 12°C, added 60ml of acetic anhydride, reacted at 15-20°C for 6 hours; Extract with dichloromethane, combine the organic layers, wash with water, separate layers, dehydrate the organic layer with anhydrous sodium sulfate; filter, and concentrate the filtrate to nearly dryness;
[0040] Add 90ml of dichloromethane to dissolve, add 150ml of n-hexane, concentrate to an appropriate amount, add 150ml of n-hexane, concentrate until a large amount of crystals precipitate, cool, filter, and obtain a wet product; dry to obtain compound 2β-(4- Morpholinyl)-16β-(1-pyrrolidinyl)-5α-androstane-3α, 17β-diol-diacetate (I) 34.1g; mp: 138~140℃, [α] D 20 =+12.7°(10mg / ml CHCl 3 ), the HPLC purity is 99.3%. ...
Embodiment 2
[0042] Preparation of compound 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androstane-3α, 17β-diol-diacetate (I) crystals:
[0043] In the reaction flask, add 120ml of dichloromethane, compound 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androstane-3α, 30g of 17β-diol (II), and stir , cooled to 8°C, added 60ml of acetic anhydride, reacted at 10-15°C for 7 hours; poured into ice water, added 180ml of dichloromethane, stirred, cooled to below 5°C, neutralized with sodium carbonate solution, layered, The layers were extracted with dichloromethane, the organic layers were combined, washed with water, separated into layers, the organic layer was dehydrated with anhydrous sodium sulfate; filtered, and the filtrate was concentrated to nearly dryness;
[0044]Add 90ml of chloroform to dissolve, add 150ml of n-heptane, concentrate to an appropriate amount, add 150ml of n-heptane, concentrate until a large amount of crystals precipitate, cool, filter, and obtain a wet product; dry to obta...
Embodiment 3
[0046] Preparation of compound 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androstane-3α, 17β-diol-diacetate (I) crystals:
[0047] In the reaction flask, add 120ml of dichloromethane, compound 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androstane-3α, 30g of 17β-diol (II), and stir , cooled to 15°C, added 60ml of acetic anhydride, reacted at 20-25°C for 5 hours; Extract with dichloromethane, combine the organic layers, wash with water, separate layers, dehydrate the organic layer with anhydrous sodium sulfate; filter, and concentrate the filtrate to about 100ml;
[0048] Add 150ml of petroleum ether, concentrate to an appropriate amount, add 150ml of petroleum ether, concentrate until a large amount of crystals precipitate, cool, filter, and obtain a wet product; dry to obtain the compound 2β-(4-morpholinyl)-16β- (1-Pyrrolidinyl)-5α-androstane-3α, 17β-diol-diacetate (I) 33.7g; mp: 138~140℃, [α] D 20 =+13.0°(10mg / ml CHCl 3 ), the HPLC purity is 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com