A kind of preparation method of rocuronium bromide intermediate
A technology for rocuronium bromide and intermediates, which is applied in the field of preparation of rocuronium bromide intermediates, can solve the problems of unindustrialization, high production cost, dark product color, etc., and reduce the loss of main products, by-products, and three wastes The effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
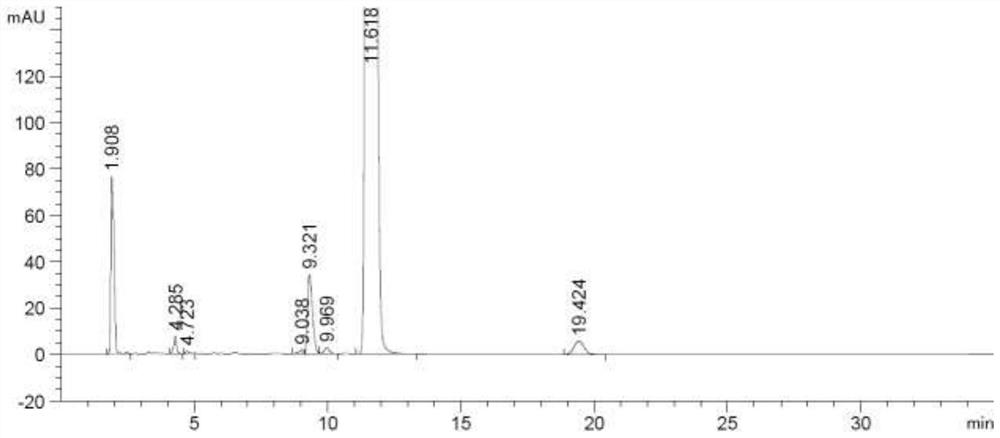
[0030] Add 15.6g (0.11mol) of phosphorus pentoxide and methanesulfonic acid (156g) to a clean 250mL three-necked flask, stir at room temperature for 2 hours, then add epiandrosterone (29.0g, 0.10mol), heat up to 110°C, and react At 6 hours, TLC showed no starting material. The reaction solution was lowered to room temperature, poured into 500 mL of water, extracted with 500 mL of dichloromethane, the organic phase was washed twice with 250 mL of 5% aqueous sodium carbonate solution, and the organic phase was washed twice with 250 mL of purified water, dried over anhydrous sodium sulfate, filtered, The dichloromethane was recovered by concentration under reduced pressure to obtain a dark brown oily substance, which was crystallized with methanol to obtain 21.6 g of off-white solids. The HPLC purity was greater than 95% and the molar yield was 79.4%. The detection chromatographic results were shown in Table 1 below. figure 1 shown.
[0031] Table 1 HPLC data table of purity of ...
Embodiment 2
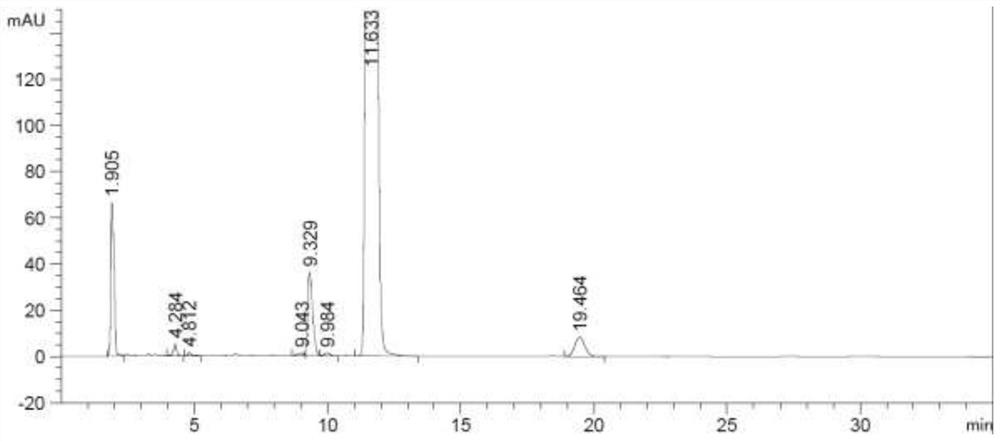
[0035] Add 15.6g (0.11mol) of phosphorus pentoxide and methanesulfonic acid (156g) into a clean 250mL three-necked flask, stir at room temperature for 2 hours, then add epiandrosterone (29.0g, 0.10mol), heat up to 40°C, and react At 17 hours, TLC showed no starting material. The reaction solution was lowered to room temperature, poured into 500 mL of water, extracted with 500 mL of dichloromethane, the organic phase was washed twice with 250 mL of 5% aqueous sodium carbonate solution, and the organic phase was washed twice with 250 mL of purified water, dried over anhydrous sodium sulfate, filtered, The dichloromethane was recovered by concentration under reduced pressure to obtain 24.3 g of off-white solid with HPLC purity of 96.7% and molar yield of 89.3%. The detection chromatographic results are shown in Table 2 below. The chromatogram is shown in figure 2 shown.
[0036] Table 2 HPLC data table of purity of 5α-androst-2-en-17-one prepared in Example 2
[0037]
Embodiment 3
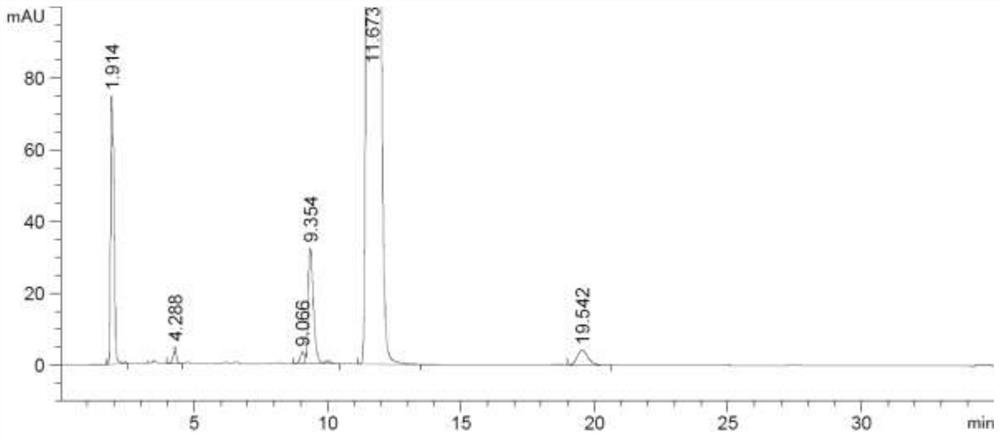
[0039] Phosphorus pentoxide (15.6g, 0.11mol), methanesulfonic acid (15.6g, 0.16mol), and dichloromethane (300mL) were added to a clean 500mL single-neck flask, stirred at room temperature for 2 hours, and then added epiandrosterone (29.0 g, 0.10 mol), the reaction was refluxed for 17 hours. The reaction solution was lowered to room temperature, poured into 500 mL of water, supplemented with 200 mL of dichloromethane, the organic phase was washed twice with 250 mL of 5% aqueous sodium carbonate solution, and the organic phase was washed twice with 250 mL of purified water, dried over anhydrous sodium sulfate, and filtered. , and concentrated under reduced pressure to recover dichloromethane to obtain 24.6 g of an off-white solid with a molar yield of 90.4%, a HPLC purity of 97.1%, and a moisture content of less than 0.1%. Its detection chromatographic results are shown in Table 3 below, and the chromatogram is shown in Table 3 below. image 3 shown.
[0040] Table 3 HPLC data...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com