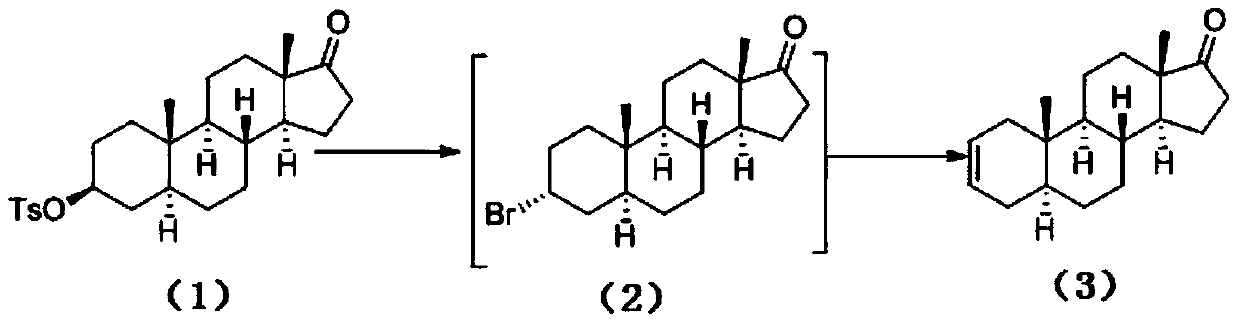
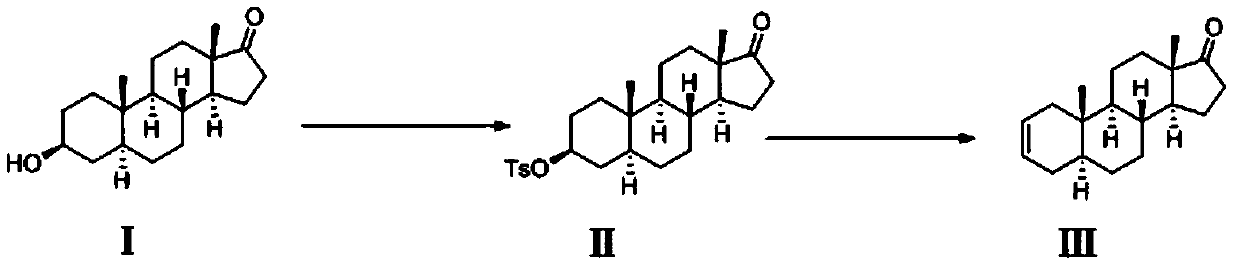
Preparation method of rocuronium bromide intermediate 5alpha-sterane-2-ene-17-one
A technology of rocuronide and an intermediate, which is applied in the field of pharmaceutical synthesis, can solve the problems of many by-products in the preparation process, cumbersome post-processing and high manufacturing cost, and achieves the effects of reducing energy consumption, shortening reaction time and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 140 grams (0.314 mol) of epiandrosterone TS, 210 grams of N,N-dimethylformamide, and 203 grams (0.628 mol) of tetrabutylammonium bromide into the reaction flask, start stirring and raise the temperature to 98 ° C for 23 hours After the reaction, the temperature was lowered to 55°C, water was added, solids were precipitated, filtered, the collected solids were stirred with water and then filtered again, the collected solids were dried to obtain 84.8 grams of 5α-stan-2-en-17-one.
Embodiment 2
[0024] Add 140 grams of epiandrosterone TS (0.314 mol), 280 grams of N,N-dimethylformamide, and 250 grams (0.785 mol) of tetrabutylammonium bromide into the reaction flask, start stirring and raise the temperature to 102°C for 24 hours After the reaction, the temperature was lowered to 60°C, water was added, solids were precipitated, filtered, the collected solids were stirred with water and then filtered again, the collected solids were dried to obtain 82.3 grams of 5α-stan-2-en-17-one.
Embodiment 3
[0026] Add 140 grams (0.314 mol) of epiandrosterone TS, 280 grams of N,N-dimethylformamide, and 220 grams (0.682 mol) of tetrabutylammonium chloride into the reaction bottle, start stirring and raise the temperature to 100 ° C for 23 hours After the reaction, the temperature was lowered to 58°C, water was added, solids were precipitated, filtered, the collected solids were stirred with water and then filtered again, the collected solids were dried to obtain 77.9 grams of 5α-stan-2-en-17-one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com