Rocuronium bromide enantiomeric impurity, or salt thereof and method for preparing rocuronium bromide enantiomeric impurity or salt
A technology of isomers and enantiomers, which is applied in the field of drug synthesis and can solve problems such as drug risks for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation of embodiment 1 formula III compound
[0037]
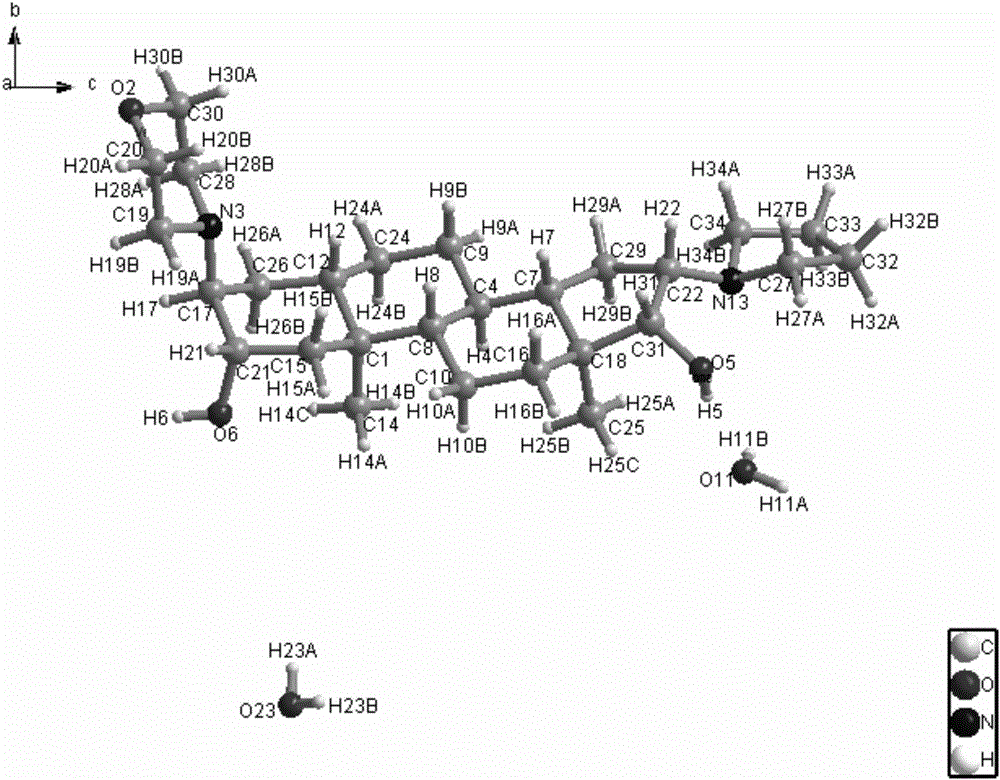
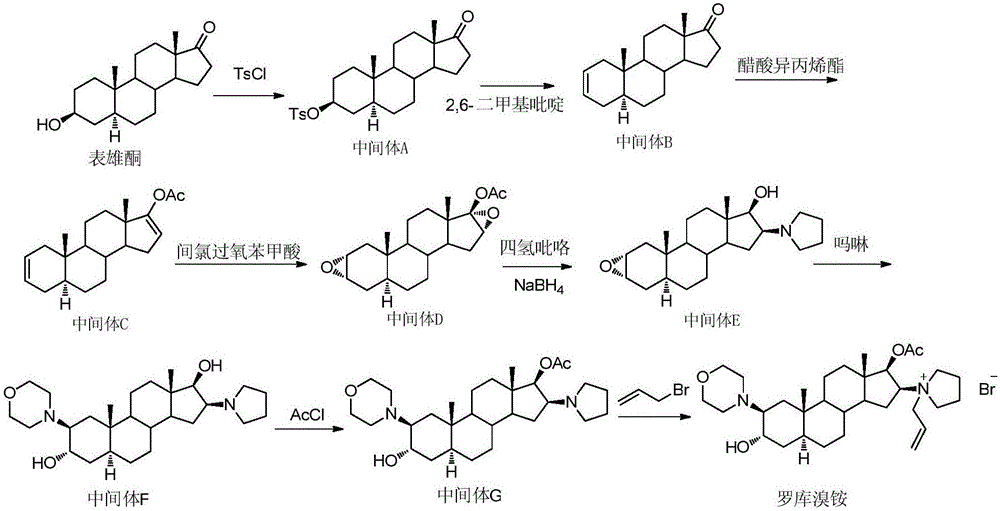
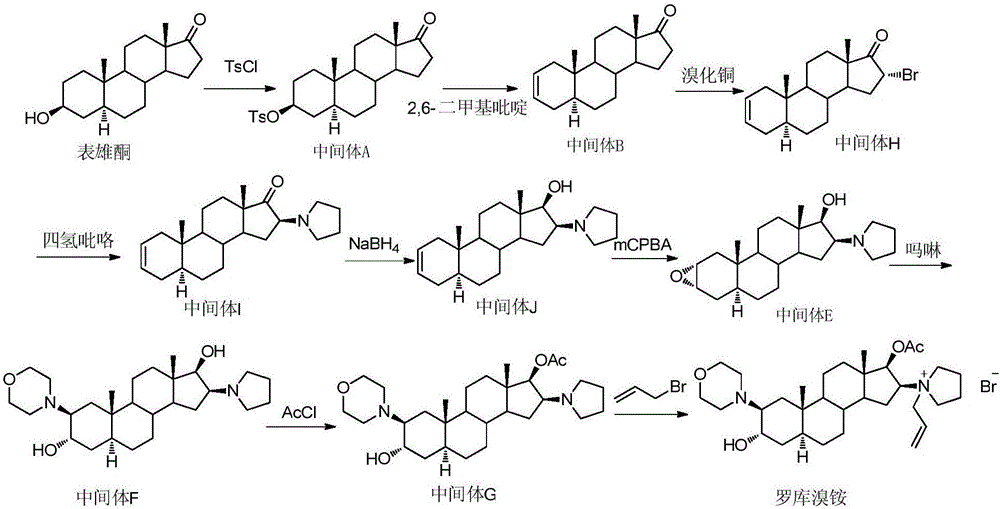
[0038] In turn, 2.3g of the compound of formula IV (prepared according to the method of (Amino-steroids. Part 6. Stereospecific Syntheses of Eight, Isomeric, Steroidal Vicinal 2,3-Amino-alcohols, J.C.S.Perkin I, 1979, 2235-2247)), 10mL of water 15mL morpholine was added in the round-bottomed flask, stirred and reacted after being heated to reflux; TLC detected that the reaction was complete. Slowly add ice water to the reaction solution to quench the reaction, extract with ethyl acetate, separate layers, wash the organic layer with water, wash with saturated sodium chloride, dry, concentrate, and column chromatography of the residue gives the compound of formula III as a light yellow solid of about 2.3 g, yield: 80.5%. The obtained compound is subjected to single crystal diffraction, and its diffraction pattern is as follows figure 1 , proving that it is the configuration shown in Formula III.
[0039...
Embodiment 4
[0048] The preparation of embodiment 4 formula I compound
[0049] Add 1.5g of the compound of formula II prepared in Example 2 or 3 and 10mL of dichloromethane into a 50mL round bottom flask in turn, add 0.6mL of allyl bromide under stirring conditions, and react at room temperature. TLC monitored the completion of the reaction. Add 20 mL of diethyl ether to the reaction, precipitate a solid, filter, wash the filter cake with diethyl ether, collect about 1.5 g of the compound of formula I as the solid, yield: 80%.
[0050] 1 H-NMR (d 6 -DMSO, 400MHz): δ=10.11(s,1H), 6.18~6.10(m,1H), 5.64~5.59(m,2H), 5.08~5.06(m,1H), 3.99~3.88(m,7H) ,3.61(m,4H),3.39~3.19(m,5H),2.18(s,3H),2.10~1.75(m,10H),1.70~1.60(m,2H),1.54~1.45(m,4H) ,1.39(m,2H),1.29~1.26(m,2H),1.11~1.04(m,2H),0.90~0.76(m,8H)ppm.
[0051] HRMS-ESI(m / z):529.4008(M–Br) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com