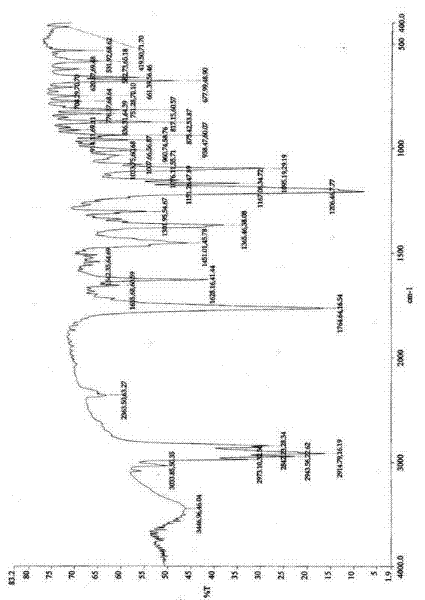
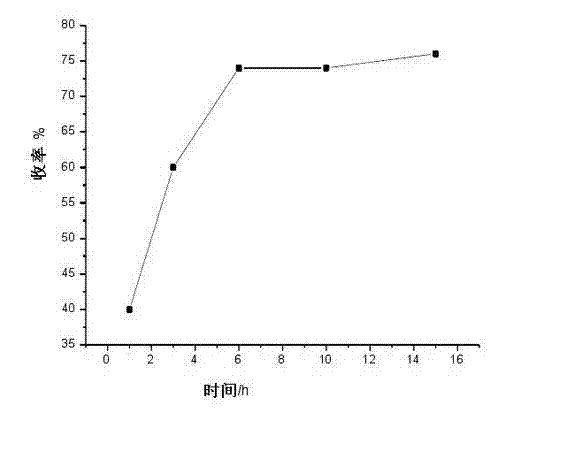
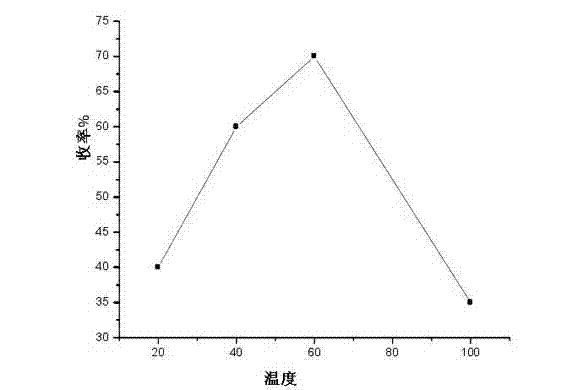
Preparation method for rocuronium bromide key intermediate 2alpha, 3alpha-epoxy-16beta-(1-pyrrolidyl)-5alpha- androstane-17 hydroxy
A technology of pyrrolidinyl and rocuronium bromide, applied in the direction of steroids and organic chemistry, can solve the problems of difficult separation of isomers, small steric hindrance, poor stereoselectivity of synthetic routes, etc., and achieve high yield High, low raw material cost, strong stereoselective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1, a key intermediate 2α,3α-epoxy-16β-(1-pyrrolidinyl)-5α-androstane- The preparation method of 17 hydroxyl groups, its synthetic steps are as follows:
[0055] (1) Using the compound 5α-androst-2-en-17one of the formula (II) as a raw material, transesterification with an enene to generate the compound 2,16-diene-17-acetyl oxide of the formula (III); the The enyl ester is selected from any one of vinyl acetate, ethylene carbonate, isopropenyl acetate, phenylpropenyl acetate, tricyclodecenyl propionate or tricyclodecenyl acetate or a mixture of any of several in any proportion ;
[0056] (2) Formula (Ⅲ) compound 2,16-diene-17-acetyl oxide is oxidized by organic peroxyacid to prepare formula (Ⅳ) 2α, 3α, 16α, 17α-diepoxide; the organic peroxy The acid is selected from the group consisting of peroxyformic acid, peroxyacetic acid, peroxytrifluoroacetic acid, peroxypropionic acid, peroxybutyric acid, peroxyisovaleric acid, long chain peroxy fatty acid, peroxybenzoic...
Embodiment 2
[0058] Embodiment 2, the concrete steps of the preparation method described in embodiment 1 are as follows:
[0059] (1) Transesterification reaction: Mix and stir 5α-androst-2-en-17one and enester, heat up to 45°C, slowly add enester solution dissolved in catalyst p-toluenesulfonic acid, dropwise, Warm up the system to reflux state within 3 hours, start slow vacuum distillation after reflux; evaporate the solvent enester to dryness within 6 hours, add ice water to the concentrated solution and stir, immediately a solid precipitates, suction filter and wash the filter with 5°C pure water Cake until neutral; dissolve the precipitated solid in ether, wash the organic phase with water to pH 6.8, combine the organic layer, add anhydrous sodium sulfate or magnesium sulfate to dry for 2.5 hours, filter with suction, add basic alumina to the filtrate, and stir at room temperature After 2h, suction filtration, the filtrate was concentrated, and a light yellow oily substance appeared, ...
Embodiment 3
[0062] Embodiment 3, the concrete steps of the preparation method described in embodiment 1 are as follows:
[0063] (1) Transesterification reaction: Mix and stir 5α-androst-2-en-17one and enester, heat up to 30°C, slowly add enester solution dissolved in catalyst p-toluenesulfonic acid, dropwise, Warm up the system to reflux within 1 hour, start slow vacuum distillation after reflux; evaporate the solvent enester to dryness within 5 hours, add ice water to the concentrated solution and stir, immediately a solid precipitates out, filter with suction and wash with pure water at 0°C Cake until neutral; dissolve the precipitated solid in ether, wash the organic phase with water to pH 7.2, combine the organic layer, add anhydrous sodium sulfate or magnesium sulfate to dry for 2 h, filter with suction, add basic alumina to the filtrate, and stir at room temperature for 1 h , filtered with suction, the filtrate was concentrated, and a light yellow oily substance appeared, and a lar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com