Processes for the synthesis of rocuronium bromide
a technology of rocuronium bromide and synthesis process, which is applied in the direction of steroids, muscular disorders, neuromuscular disorders, etc., can solve the problems of increasing production costs, reducing yield, and increasing manufacturing tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
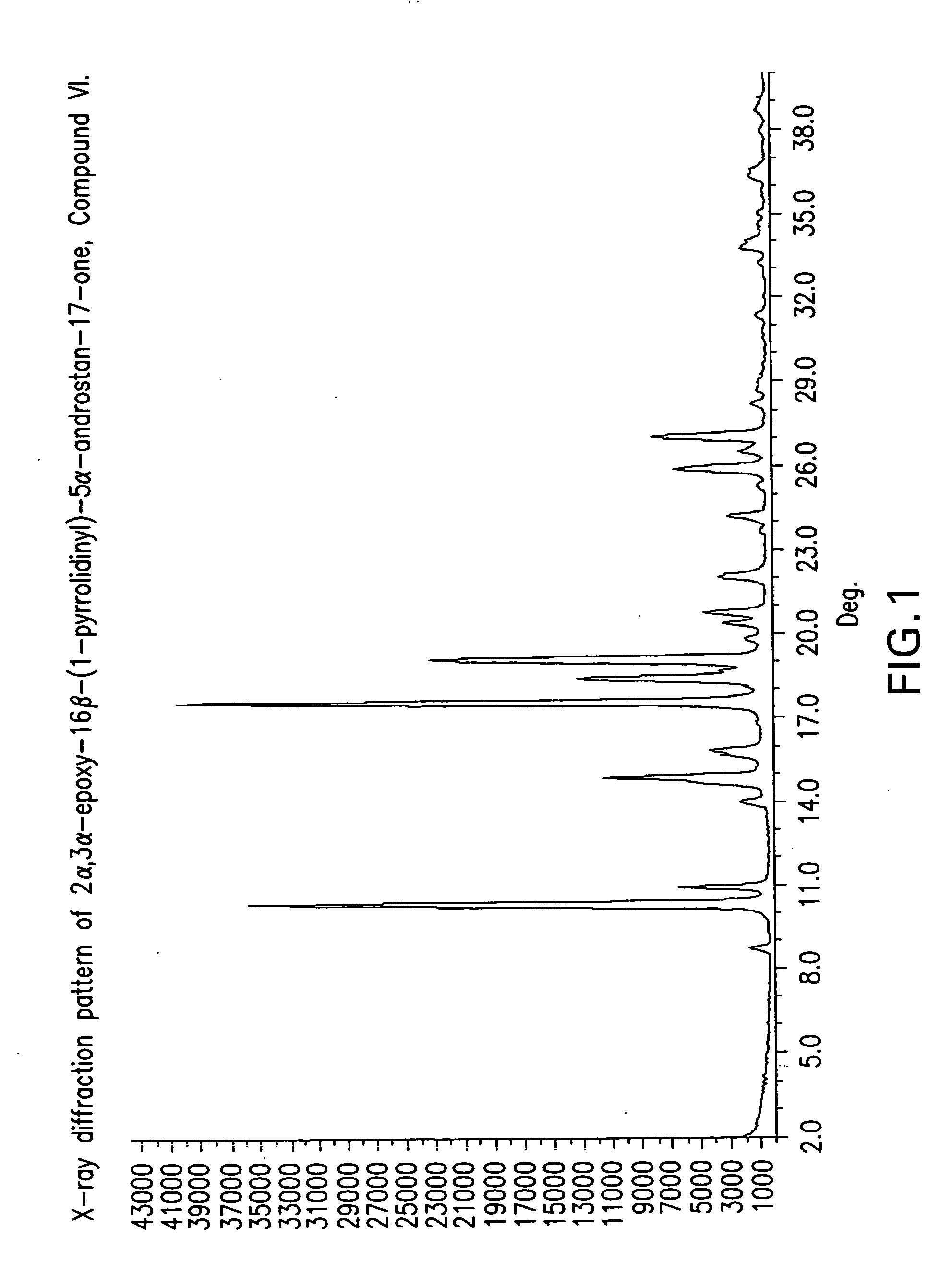
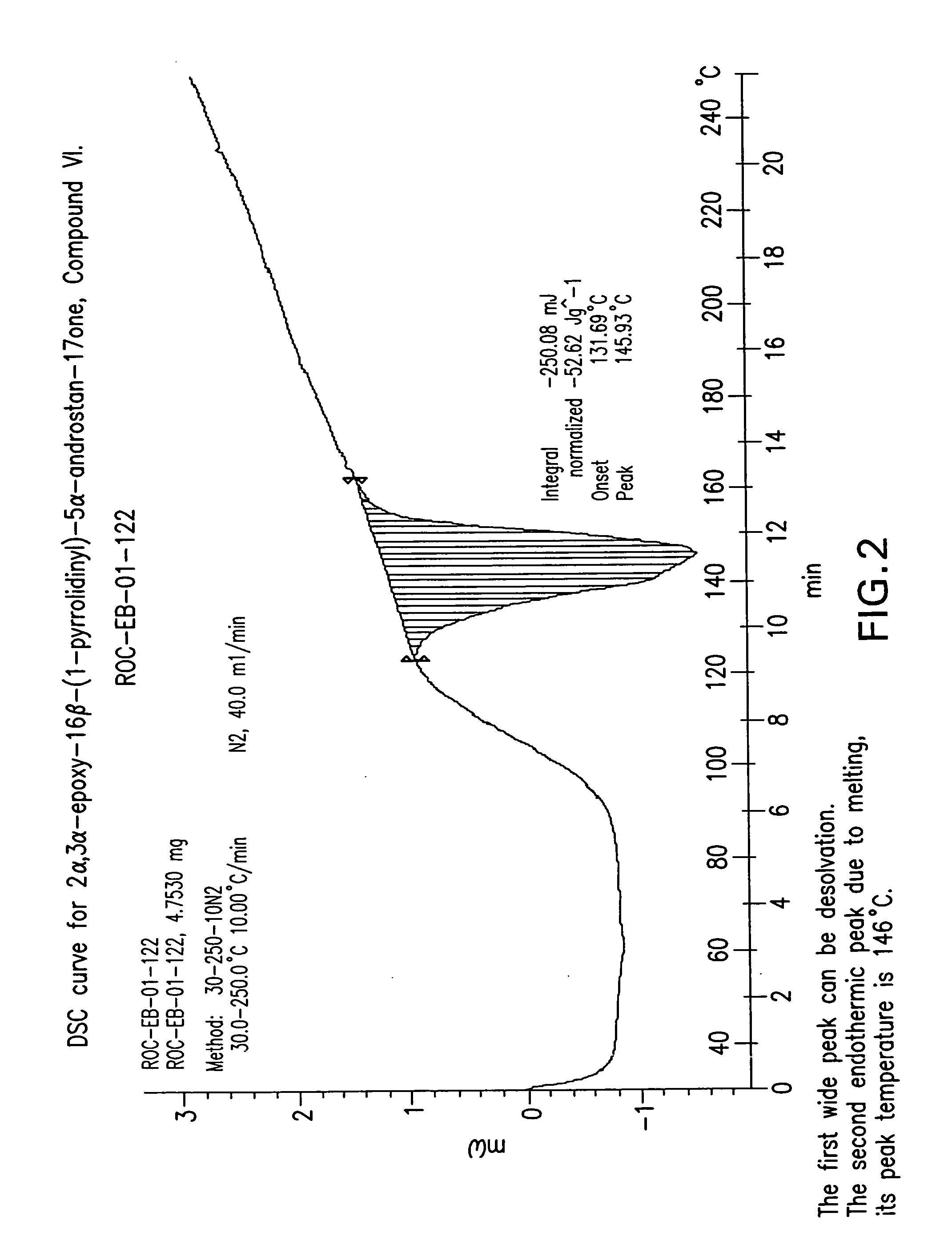
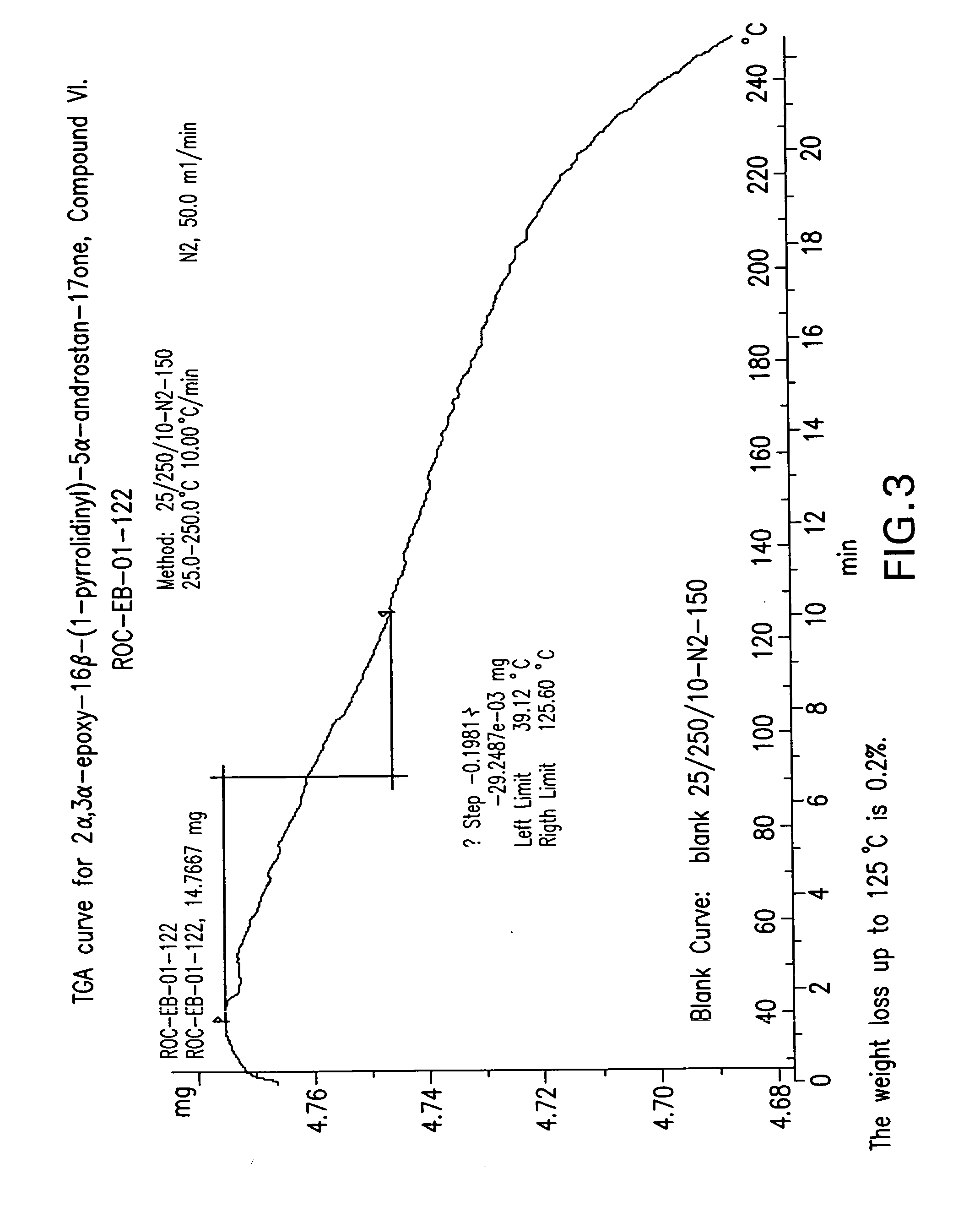
Preparation of the mixture of 2α,3α-epoxy-16β-(1-pyrrolidinyl)-5α-androstan-17-one, Compound VI, and 2α,3α-epoxy-16α-(1-pyrrolidinyl-5α-androstan-17-one, Compound VI-a
[0120]To a solution of 2α,3α,16α,17α-bisepoxy-5α-androstan-17β-ol acetate III (50 g, 144.32 mmol) in methanol (500 mL) was added a 4 N solution of sodium hydroxide (40 mL, 9.525, 158.75 mmol) at 20-24° C. and under nitrogen atmosphere. The mixture was heated to reflux (60-65° C.) for 30 min, followed by cooling to 40° C. and addition of pyrrolidine (24 mL, 288.64 mmol). The reaction mixture was heated to reflux (60-65° C.) for 30-45 min, followed by cooling to room temperature and then ice-water (500 mL) was added, to obtain a suspension. The suspension was stirred at 5° C. for 30 min and then the solid was filtered off and washed with cold water (2×200 mL). The wet solid was dried under vacuum to give 47 g of Compound VI as a pale yellow powder having a ratio of VI-a to VI of 18:82.
example 2
Equilibration process to obtain 2α,3α-epoxy-16β-(1-pyrrolidinyl)-5α-androstan-17-one, Compound VI
[0121]The dried compound of Example 1 (47 g) was suspended in methanol (235 mL) and refluxed (60-65° C.) between 20-30 min. Water was added (715 mL) over a period of 20 min. and the resulting mixture was heated at 65-70° C. during 30 min. The formed suspension was cooled at 0-5° C. in a period of 30-40 min and the suspension was further stirred at this temperature for 20 min. The suspension was filtered and washed with water (188 mL). The wet solid was dried under vacuum to give 43 g (120.26 mmol, 83% yield, purity of 93% by HPLC, having a melting point of 146° C.) of Compound VI as a white solid, having a ratio of VI-a to VI of 1.8:98.2. [α]D20+101.1 (c=1.0 in CHCl3).
example 3
Preparation of 2α,3α-epoxy-16β-(1-pyrrolidinyl)-5α-androstan-17β-ol, Compound IV
[0122]A suspension of 1 g (2.8 mmol) of Compound VI in of methanol (20 mL) was cooled to −10° C., and then sodium borohydride (200 mg, 5.04 mmol) was added carefully in portions, under a nitrogen atmosphere. The resulting heterogeneous reaction mixture was allowed to reach 20-24° C. and stirred for at least 3 h. The reaction mixture was diluted with CH2Cl2 and then purified water was added at 20-22° C. The solution was stirred for 10 min. and then the phases were separated. The organic phase was washed with purified water and the solution was concentrated under vacuum to give the desired crude Compound IV. The product was dried in a vacuum oven at 40° C. for at least 16 h to give 1 g (0.00278 mole) of a white solid (99.3% yield) containing the product IV 98.5% and the isomer IV-a 1.1% area by HPLC. M.P. 171° C.; [α]D20+34.0 (c=1.0 in CHCl3). The structure was confirmed by spectroscopic analysis.
[0123]Cha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com