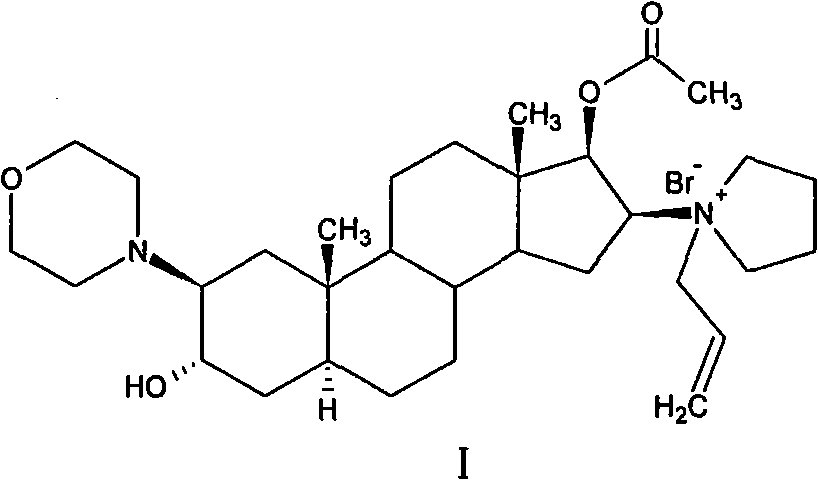
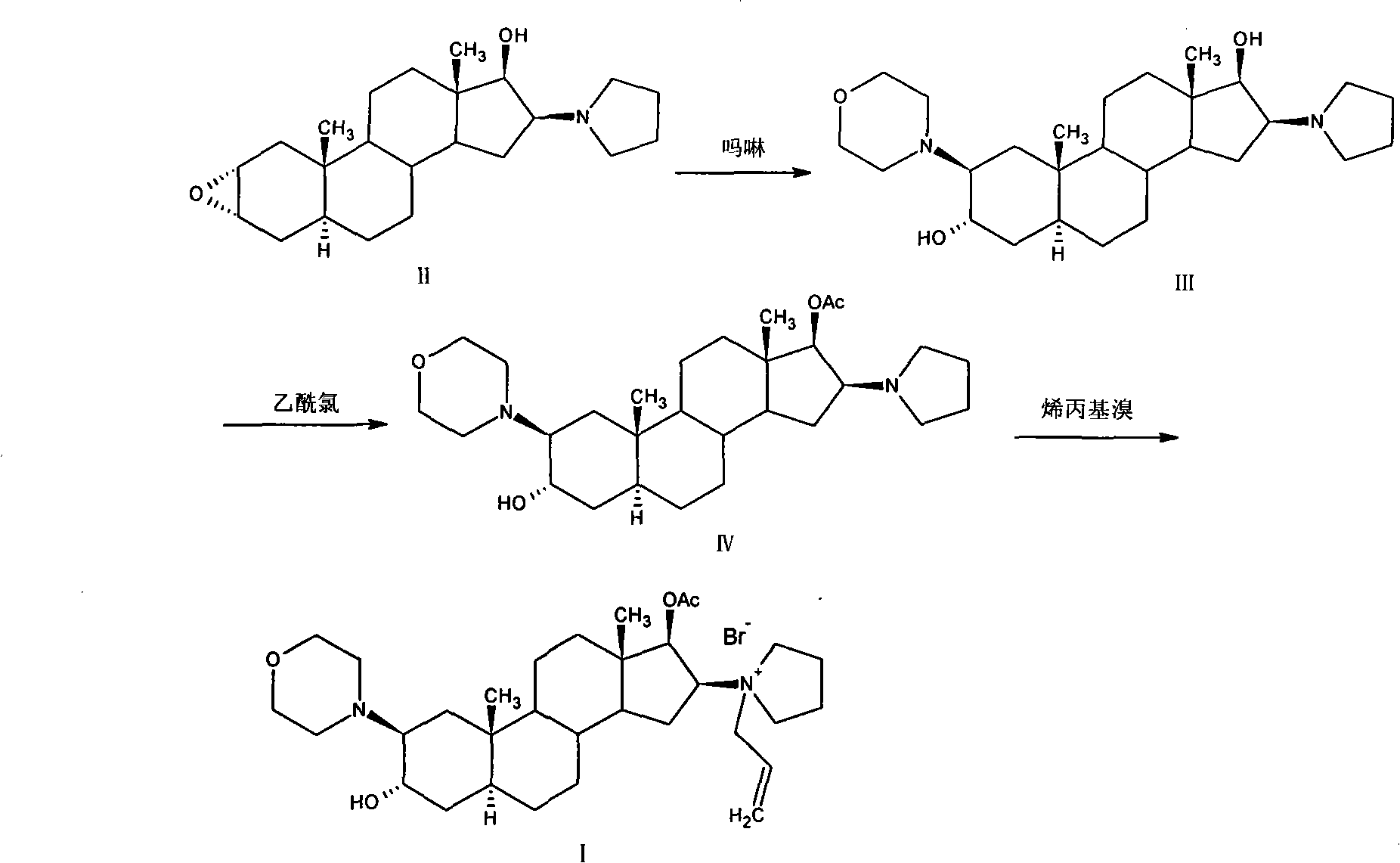
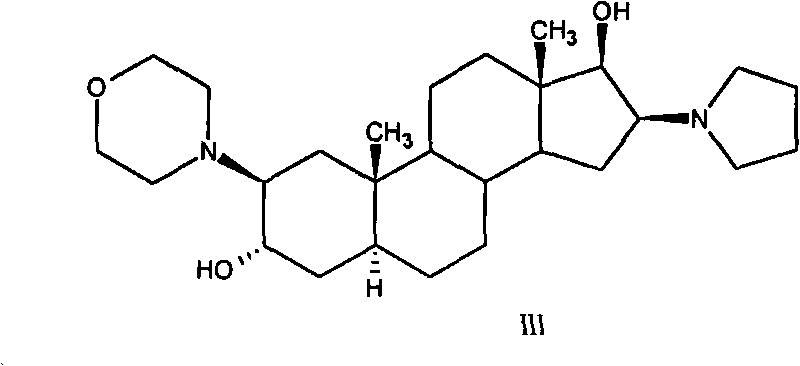
High-purity (2 beta, 3 alpha, 5 alpha, 16 beta, 17 beta)-2-(4-morpholinyl)-16-(1-pyrrolidinyl)-androstane-3,17-diol or composition thereof and preparation method thereof
A pyrrolidinyl, high-purity technology, applied in the directions of steroids, drug combinations, muscular system diseases, etc., can solve problems such as unobtainable yield, subsequent process influence, and purity description of the compound of formula III
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0141] Embodiment 1a: the HPLC analysis method of formula III compound
[0142] Get appropriate amount of this product, add the acetonitrile solution of 0.2% trifluoroacetic acid to make the solution containing about 7.0mg in every 1ml, as need testing solution, measure according to high performance liquid chromatography (Chinese Pharmacopoeia 2005 edition two appendix VD). Use silica gel as a filler, use 0.025mol / L tetramethylammonium hydroxide solution (take 4.53g of tetramethylammonium hydroxide pentahydrate, add 900ml of water to dissolve, adjust the pH value to 7.4 with phosphoric acid, dilute to 1000ml with water, Shake well to obtain)-acetonitrile (10:90) as the mobile phase, the detection wavelength is 210nm, and the column temperature is 30°C. The number of theoretical plates should be no less than 2000 based on the peak of the compound of formula III. Precisely measure 10ul of the test solution, inject it into the liquid chromatograph, record the chromatogram to 3 t...
Embodiment 1b
[0143] Embodiment 1b: the HPLC analysis method of formula IV compound
[0144] Take an appropriate amount of this product, add 90:10 (acetonitrile-water) to make a solution containing about 3.0 mg per 1 ml, as the test solution, and measure it according to high performance liquid chromatography (Chinese Pharmacopoeia 2005 edition two appendix VD). Use silica gel as a filler, use 0.025mol / L tetramethylammonium hydroxide solution (take 4.53g of tetramethylammonium hydroxide pentahydrate, add 900ml of water to dissolve, adjust the pH value to 7.4 with phosphoric acid, dilute to 1000ml with water, Shake well to obtain)-acetonitrile (25:75) as the mobile phase, the detection wavelength is 210nm, and the column temperature is 30°C. The number of theoretical plates should be no less than 2000 according to the formula IV compound peak. Precisely measure 10ul of the test solution, inject it into the liquid chromatograph, record the chromatogram to 3 times of the retention time of the ...
Embodiment 2
[0146] Embodiment 2: the preparation of formula III compound
[0147] Suspend 120g of the crude compound of formula III in 600ml of chloroform, raise the temperature to reflux, stir until the solid is completely dissolved, add 480ml of acetone to obtain a suspension, cool to 0°C to 5°C within 1 hour, and stir for 1 hour. After filtering, the filter cake was washed once with 100 ml of acetone, and dried under vacuum at 50° C. for 14 hours to obtain 115.2 g of the compound of formula III. Yield: 96%, m.p. 220°C to 222°C, [α] D 20 +86.8°(c=1.02in CHCl 3 ). HPLC purity: 99.2% compound of formula III, 0.15% compound of impurity formula III-a, 0.10% compound of impurity formula III-b, 0.05% compound of impurity formula III-c, 0.08% compound of impurity formula II.
[0148] MH in mass spectrum [ESI-MS, m / z] + The peak is 447.
[0149] The main absorption peak of the infrared spectrum measured by the KBr tablet method is 3427cm -1 、2948cm -1 、2910cm -1 、2796cm -1 、1121cm -1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com