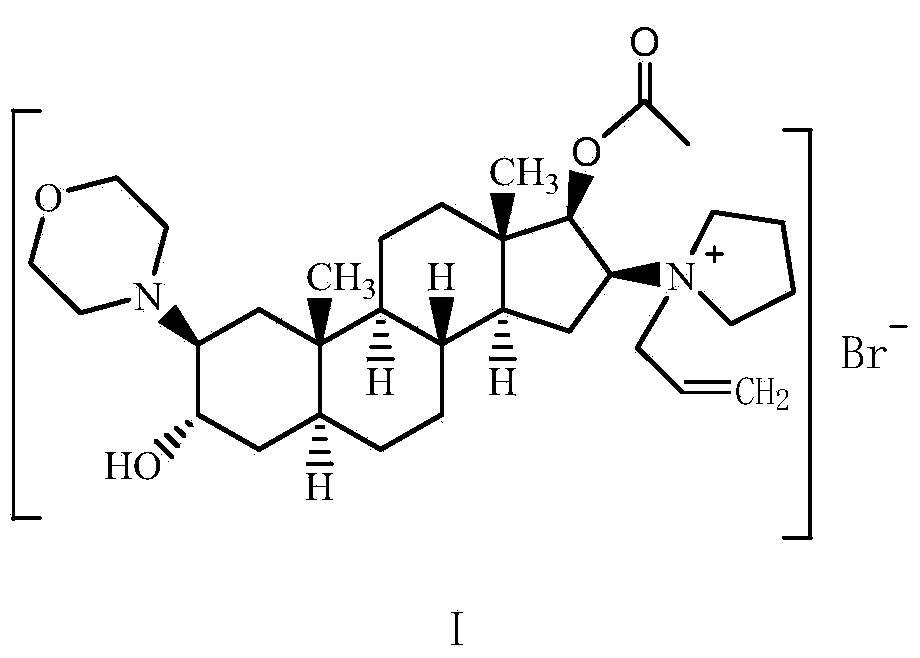
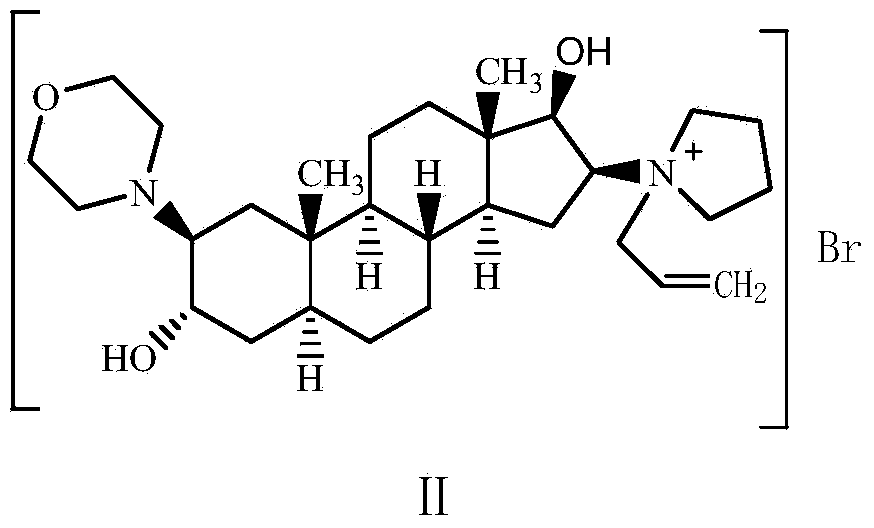
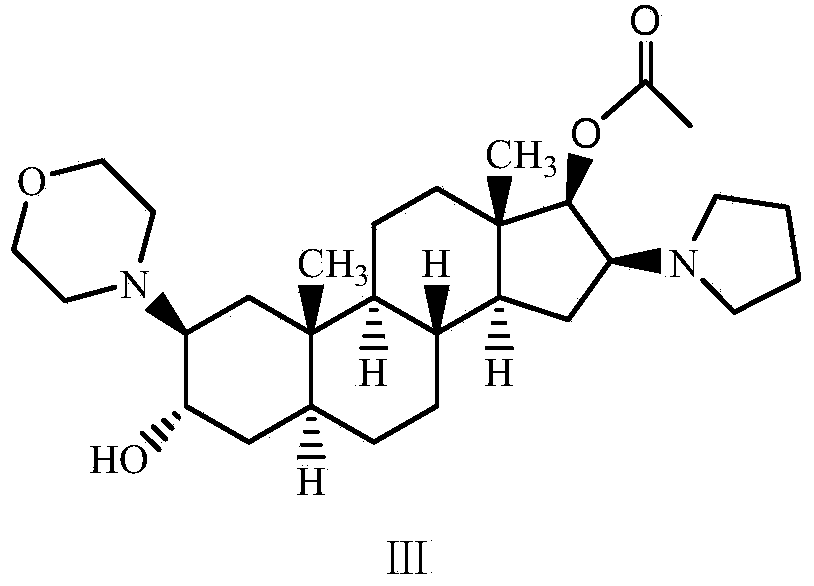
Preparation method of high-purity high-stability rocuronium bromide
A high-stability, high-purity technology, applied in the field of medicinal chemistry, can solve the problems of long operation time, difficult operation, and inability to completely remove 3-bromopropene, and achieve high stability and reduce the generation of impurity C.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of crude rocuronium bromide: with reference to Patent US2006 / 0058275 Example 3, the raw material (2β, 3α, 5α, 16β, 17β)-2-(4-morpholino)-16-(1-pyrrolyl)-androgen Add ster-3,17-diol-17-acetate (30g) and 3-bromopropene (78ml) into 120ml of acetonitrile, stir and react at room temperature for 3 hours, after the reaction, slowly add the reaction solution to 720ml Then, filter the precipitated rocuronium bromide and dry it under vacuum at 60°C overnight. After testing, the impurity A content in the crude rocuronium bromide was 0.08%, the impurity C was not detected, the total impurity was 0.35%, the moisture was 3.25%, the acetonitrile residue was 1700ppm, the ether residue was 6600ppm, and the 3-bromopropene residue was 473ppm.
[0036] Step 1: Dissolve 10.0 g of the obtained crude product in 80 ml of dichloromethane, add 8.0 g of alumina, stir vigorously for 2 hours, first filter it with filter paper, then filter it with an organic filter membrane, add the obta...
Embodiment 2
[0043] The rocuronium bromide crude product that makes in embodiment 1 is used for embodiment 2.
[0044] Step 1: Dissolve 10.0 g of the obtained crude product in 80 ml of dichloromethane, add 10.0 g of alumina, stir vigorously for 4 hours, then filter once with filter paper, and then filter again with organic filter membrane, add the obtained filtrate dropwise to 500 ml and stir vigorously In ether, filter under nitrogen protection;
[0045] Step 2: Add 100ml of deionized water to a 500ml single-mouth bottle, carefully add 10% dilute acetic acid to adjust the pH of the solution to 3.8, then add the obtained filter cake to the above solution, shake to dissolve, pour it into a petri dish, and put the petri dish Put it into a freeze-drying box and cool it down to below -45°C within 2 hours to make it freeze quickly. Vacuumize to make the atmospheric pressure in the box reach 2.66pa within 30 minutes. Insulated and vacuum-dried for 48 hours until the moisture content was ≤1.0%,...
Embodiment 3
[0051] The rocuronium bromide crude product that makes in embodiment 1 is used for embodiment 2.
[0052] Step 1: Dissolve 10.0 g of the obtained crude product in 80 ml of dichloromethane, add 18.0 g of alumina, stir vigorously for 4 hours, then filter once with filter paper, then filter again with organic filter membrane, add the obtained filtrate dropwise to 500 ml and stir vigorously In ether, filter under nitrogen protection;
[0053] Step 2: Add 100ml of deionized water to a 500ml single-mouth bottle, carefully add 10% dilute acetic acid to adjust the pH of the solution to 3.8, then add the obtained filter cake to the above solution, shake to dissolve, pour it into a petri dish, and put the petri dish Put it into a freeze-drying box and cool it down to below -45°C within 2 hours to make it freeze quickly. Vacuumize to make the atmospheric pressure in the box reach 6.20pa within 30 minutes. Insulated and vacuum-dried for 48 hours until the moisture content was ≤1.0%, 8.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com