Preparation of rocuronium
A technology of rocuronium bromide and pyrrole bromide, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of low yield and achieve the effects of improving product quality, avoiding side reactions, and avoiding epoxy ring opening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
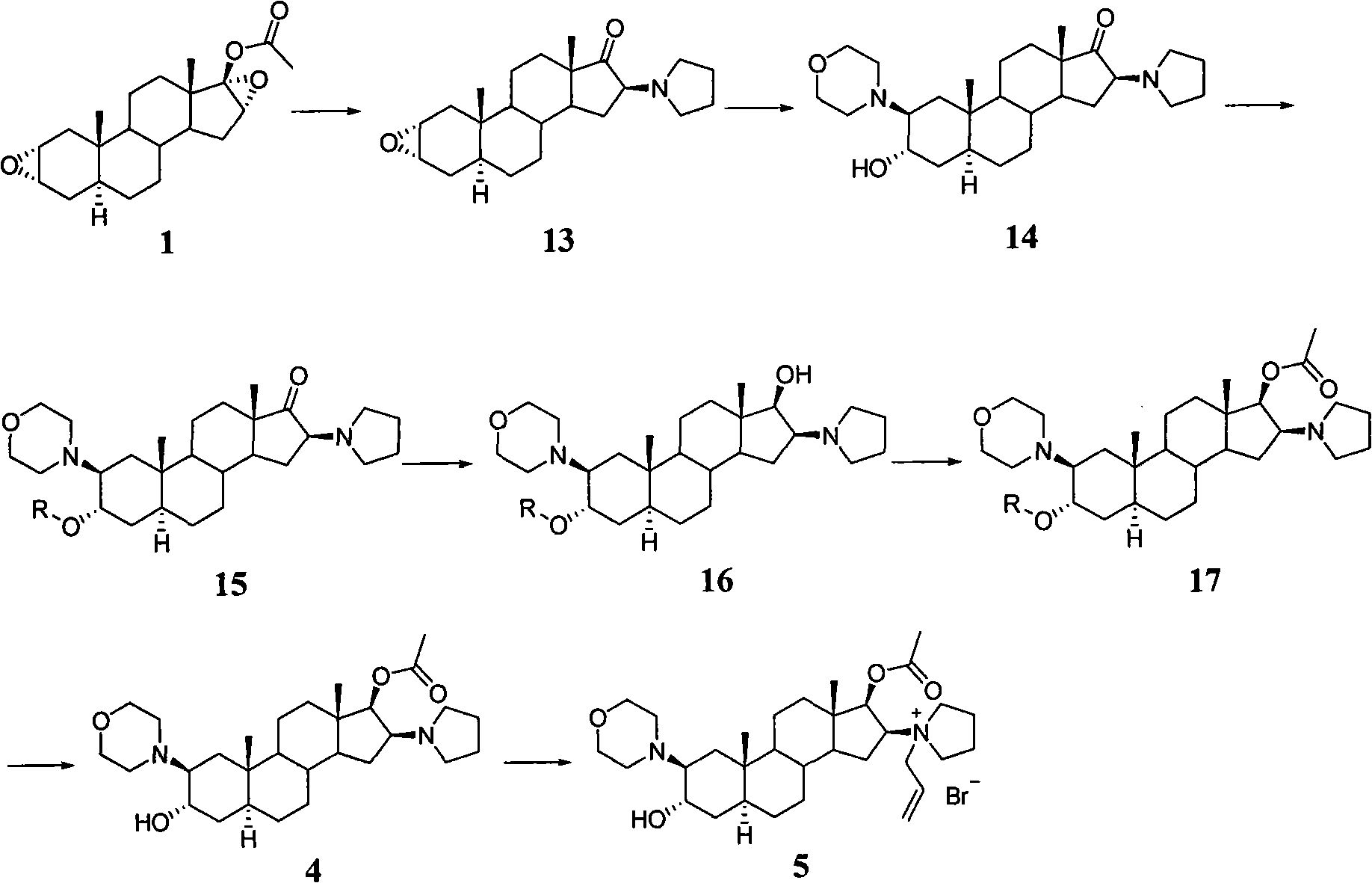
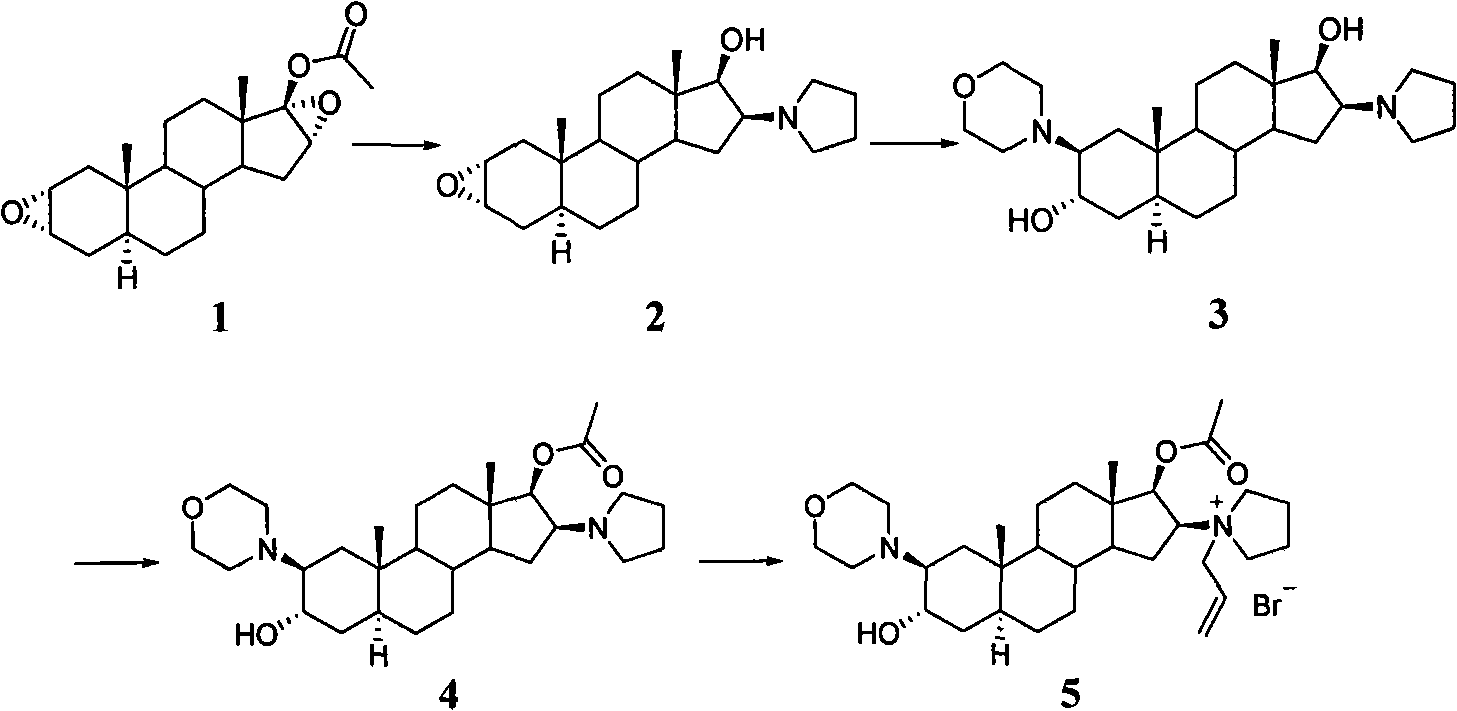
[0030] Preparation of 2α,3α-epoxy-16β-(1-tetrahydropyrrolyl)-5α-androstan-17-one
[0031] Sodium hydroxide solution (30ml; 4N) was added to 2α, 3α, 16α, 17α-diepoxy-5α-androstane-17β-ol acetate (30g) in methanol (500ml) solution, at 8-10°C Stir for 1 hour. Tetrahydropyrrole (45ml) was added, followed by reflux for 20 minutes. The reaction solution was cooled to about 10°C, added water (600ml), filtered, washed with water (3×200ml), dried, and recrystallized with acetone to obtain white crystals 2α, 3α-epoxy-16β-(1-tetrahydropyrrolyl )-5α-androstan-17-one (21 g).
Embodiment 2
[0033] Preparation of 3α-hydroxy-2β-(4-morpholinyl)-16β-(1-tetrahydropyrrolyl)-5α-androstan-17-one
[0034]Water (12ml) was added to a solution of 2α,3α-epoxy-16β-(1-tetrahydropyrrolyl)-5α-androstan-17-one (20g) in morpholine (120ml) and heated to reflux for 3 days. Poured into water (1000ml), filtered, washed with water, dried to obtain the crude product, and recrystallized from acetone to obtain pure 3α-hydroxy-2β-(4-morpholinyl)-16β-(1-tetrahydropyrrolyl )-5α-androstan-17-one (15 g).
Embodiment 3
[0036] Preparation of 3α-hydroxy-2β-(4-morpholinyl)-16β-(1-tetrahydropyrrolyl)-5α-androstan-17-one 3-O-tetrahydropyran-2-ether
[0037] Add p-toluenesulfonic acid (0.598g) in dichloromethane (130ml), stir at room temperature for 10 minutes, add 3α-hydroxyl-2β-(4-morpholinyl)-16β-(1-tetrahydropyrrolyl)-5α -Androstan-17-one (13g), after complete dissolution, add 2,3-dihydro-4H-pyran (4.84g) under stirring, and reflux for 48 hours. After completion of the reaction, cool to room temperature, add 5% sodium carbonate aqueous solution (26ml), stir until alkaline, wash with water three times (20ml×3), then wash once with 5% sodium carbonate aqueous solution. Concentrate under reduced pressure until there is almost no solvent, pour into methanol (pH7.5~8), continue to concentrate until almost no solvent, pour into methanol (pH7.5~8), continue to concentrate until almost no solvent, pour into methanol (13ml), cool overnight, Filter, wash with ice methanol, drain, and wash with water un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com