Method for preparing rocuronium bromide injection
A technology of rocuronium bromide and injection, which is applied in the field of preparation of rocuronium bromide injection, can solve the problems of high manufacturing cost and long process flow of freeze-dried preparations, and achieve low manufacturing cost, stable quality and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
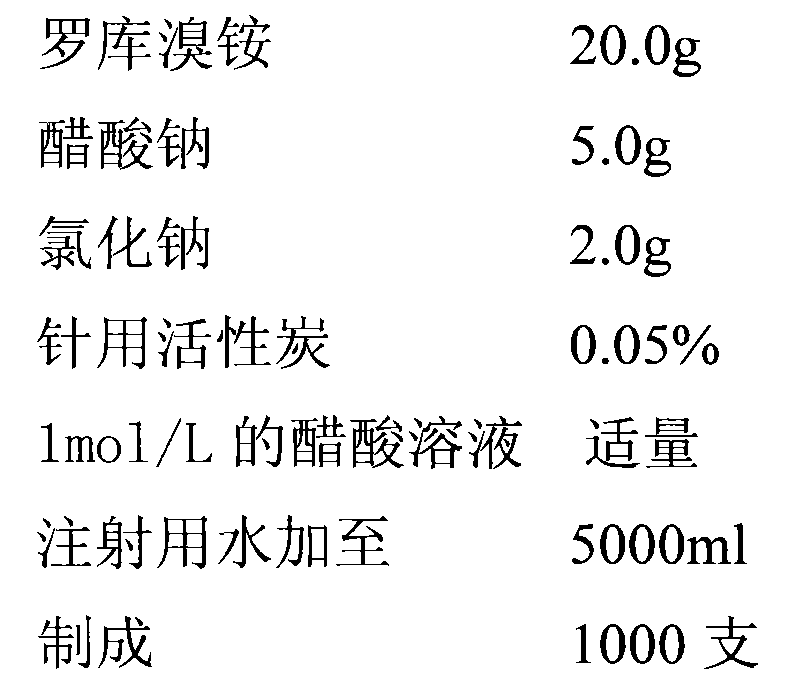
[0027] (a) take by weighing rocuronium bromide 40.0g, sodium acetate 15.0g, sodium chloride 0.8g;
[0028] (b) cooling the water for injection to room temperature;
[0029] (c) preparing acetic acid-sodium acetate buffer solution (pH3.8);
[0030] (d) Measure 3000ml of water for injection cooled to room temperature, add acetic acid-sodium acetate buffer, and mix well;
[0031] (e) Add sodium chloride, stir until completely dissolved; put rocuronium bromide into it, stir to dissolve; adjust the pH value of the solution to 4.0 with 1mol / L acetic acid;
[0032] (f) Add 2 g of activated carbon, stir at room temperature for 30 minutes, and measure the content of the decarbonized filtration intermediate; add water for injection according to the results, so that the marked content is 100%;
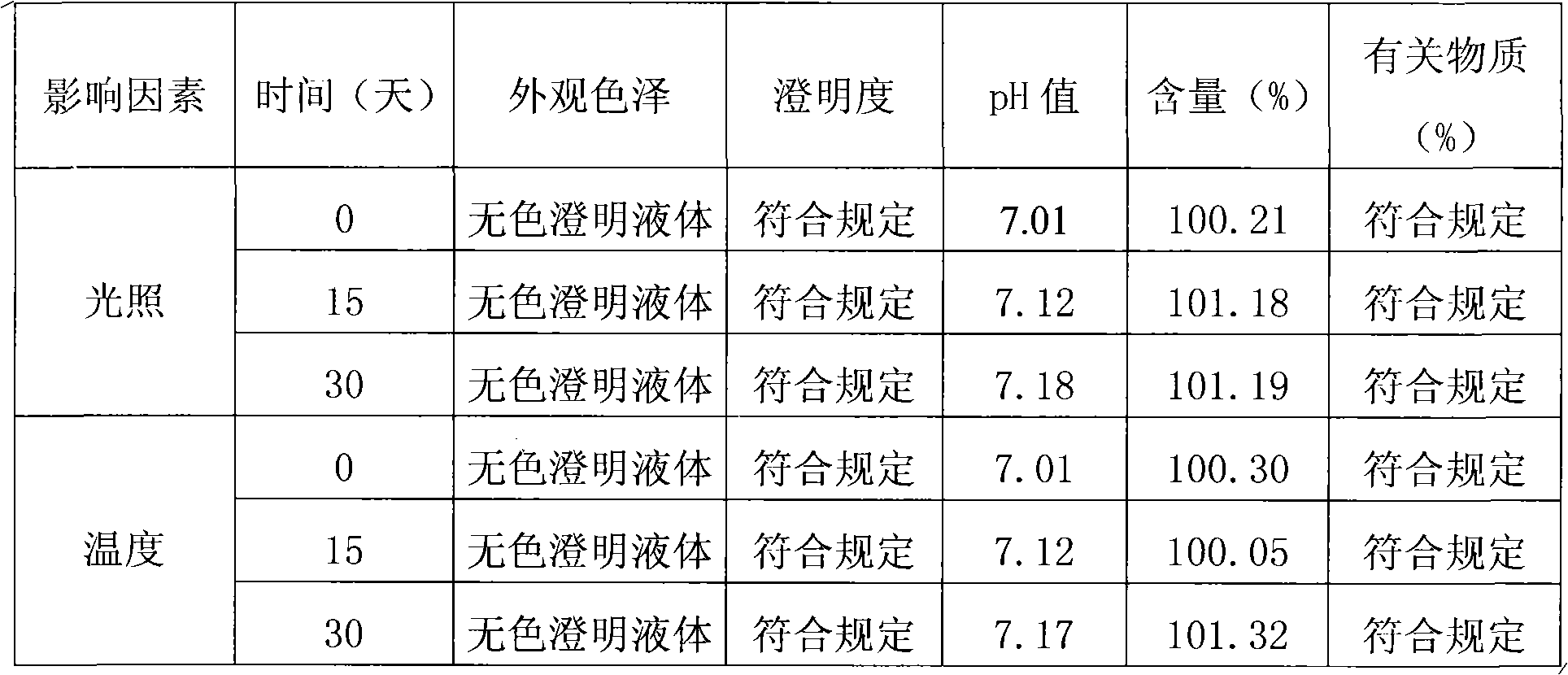
[0033] (g) Sterilize by filtration through a 0.22 μm microporous membrane; dispense into controlled antibiotic bottles, 5ml (2.5ml) per bottle, press rubber stoppers, and roll aluminum caps; st...
Embodiment 2
[0035] (a) take by weighing rocuronium bromide 50.0g, sodium acetate 10.0g, sodium chloride 1g;
[0036] (b) cooling the water for injection to room temperature;
[0037] (c) preparing acetic acid-sodium acetate buffer solution (pH3.8);
[0038] (d) Measure 3000ml of water for injection cooled to room temperature, add acetic acid-sodium acetate buffer, and mix well;
[0039] (e) Add sodium chloride, stir until completely dissolved; add rocuronium bromide, stir to dissolve; adjust the pH value of the solution to 4.0 with 1mol / L acetic acid;
[0040] (f) Add 1 g of activated carbon, stir at room temperature for 30 minutes, measure the content of the decarbonized filtration intermediate; add water for injection according to the results, so that the marked content is 100%;
[0041] (g) Sterilize by filtration through a 0.22 μm microporous membrane; dispense into controlled antibiotic bottles, 5ml (2.5ml) per bottle, press rubber stoppers, and roll aluminum caps; sterilize by aut...
Embodiment 3
[0043]
[0044] Pretreatment includes: a, cooling water for injection to room temperature; b, preparation of acetic acid-sodium acetate buffer (pH3.8); c, measuring 3000ml of water for injection cooled to room temperature, adding acetic acid-sodium acetate buffer, mixing Evenly; d, add sodium chloride, stir until completely dissolved; e, add rocuronium bromide to the above solution, stir to dissolve; adjust the pH value of the solution to 4.0 with 1mol / L acetic acid; f, add activated carbon, room temperature Stir for 30 minutes, decarbonize and filter; g, take a sample to measure the content of the intermediate; add water for injection according to the result to make the content 100%; h, filter and sterilize through a 0.22 μm microporous membrane; fill and sterilize : Divided into regulated antibiotic bottles, 5ml per bottle, fully pressed rubber stopper, rolled aluminum cap; sterilized by autoclaving at 121°C for 20min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com