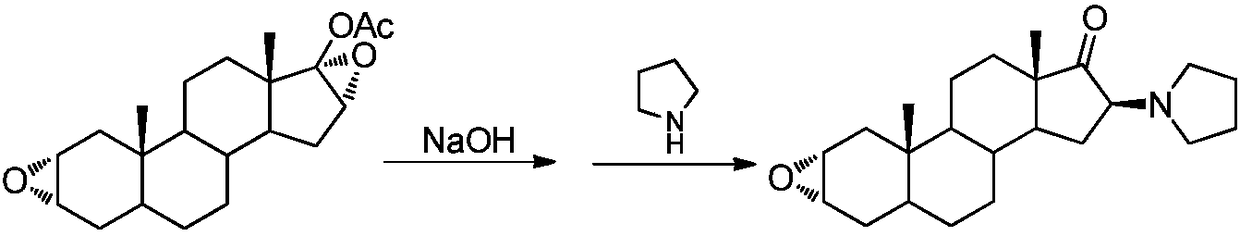
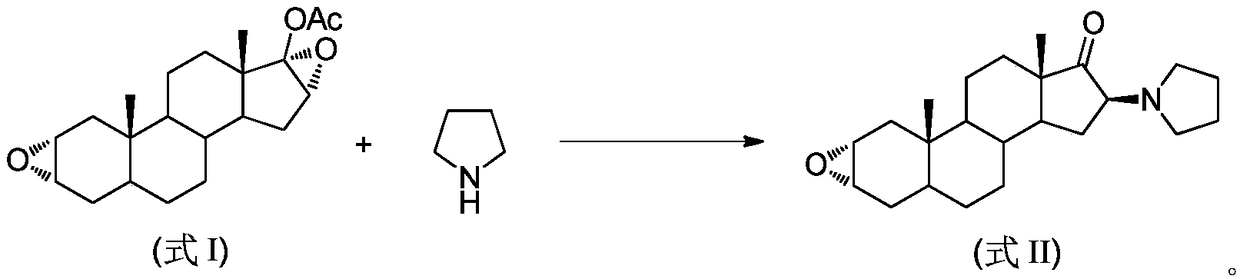
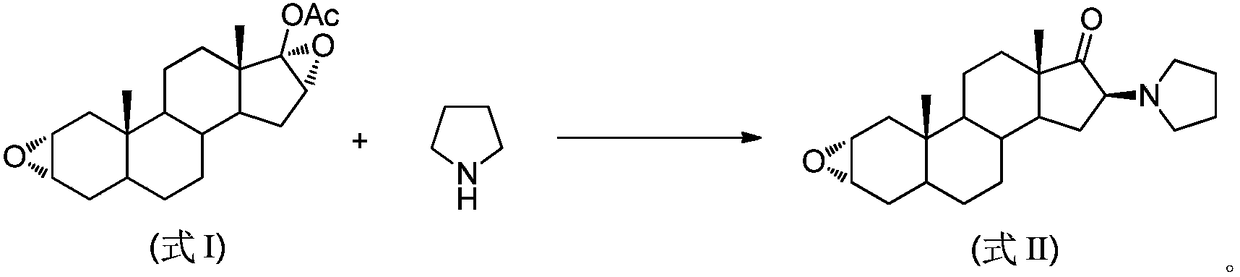
Synthesis method of 16beta-tetrahydropyrrolyl androstan-2alpha-epoxy-17-one
A technology of tetrahydropyrrolyl androstane and acetoxyandrostane is applied in the field of preparation of 16β-tetrahydropyrrolyl androst-2α-epoxy-17-one, and can solve the problems of poor selectivity, difficult separation of impurities and waste liquid volume and the like , to achieve the effect of easy operation, reducing the amount of three wastes, and reducing the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 3.46g (10mmol) 17-acetoxyandrosta-2α, 16α-diepoxide, 6g (50mmol) pyrrolidine and 17.3g silica gel to the ball mill jar, set the operating frequency of the ball mill to 15Hz, and stop the mechanical grinding after 20 minutes . The entire reaction mixture was transferred from the grinding jar to a 50 mL beaker, and 20 mL of dichloromethane was added to soak for 1 hour. After filtering and concentrating the filtrate to dryness, add a mixed solvent of methanol and water (10:1) for recrystallization to obtain 3.21 g of 16β-tetrahydropyrrolylandrost-2α-epoxy-17-one as a white solid, with a yield of 90% .
[0030] After testing, the specific characteristics of the product are as follows:
[0031] Melting point: 167.2-168.9℃, 1 H NMR (400MHz, CDCl 3 )δ3.18-3.12(m,2H),2.94-2.90(m,1H),2.78-2.72(m,2H),2.62-2.60(m,2H),2.17-2.08(m,1H),1.96- 1.91(m,2H),1.85-1.71(m,5H),1.67-0.95(m,13H),0.91(s,3H),0.80(s,3H),0.75-0.68(m,1H). 13 C NMR (100MHz, CDCl 3 ) 218.7, 69.1, 54.0, 52.3...
Embodiment 2
[0033] According to the method and steps of Example 1, the only difference is that in step (1), the molar ratio of 17-acetoxyandrost-2α,16α-diepoxide to pyrrolidine was adjusted to 1:3, and the yield was 78%.
Embodiment 3
[0035] According to the method and steps of Example 1, the only difference is that in step (1), the molar ratio of 17-acetoxyandrost-2α,16α-diepoxide to pyrrolidine was adjusted to 1:8, and the yield was 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com