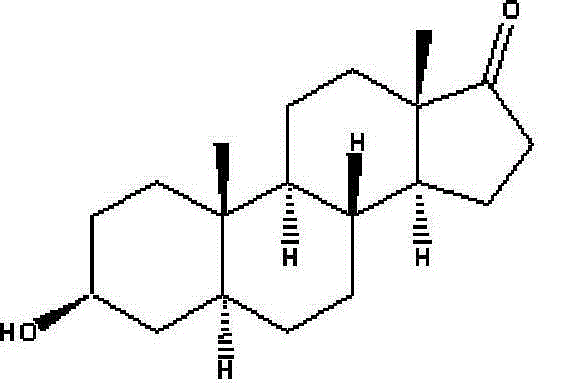
A kind of production method of synthesizing epiandrosterone by monoenolone acetate
A technology of monoenolone acetate and production method, which is applied in the chemical industry, can solve the problems of incomplete reaction of intermediate products, harsh hydrogenation reaction conditions, and many by-products, and achieve simple reaction process, simple preparation method, and low production of by-products. little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
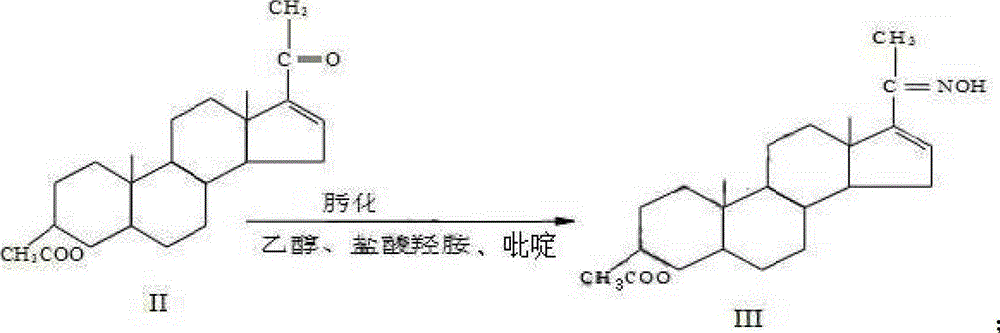
[0029] 1) Preparation of Ketoxime III
[0030] Add 300ml of ethanol, 48ml of pyridine, 24g of hydroxylamine hydrochloride, and 80g of monoenolone acetate II to the reaction flask in sequence, heat up, and time the reflux reaction for 4 hours. After the reflux is completed, cool down to 0°C and separate by suction filtration until no liquid flows out. Rinse with ethanol, then wash with water until neutral, filter and dry with suction to obtain ketoxime III 78.4g.
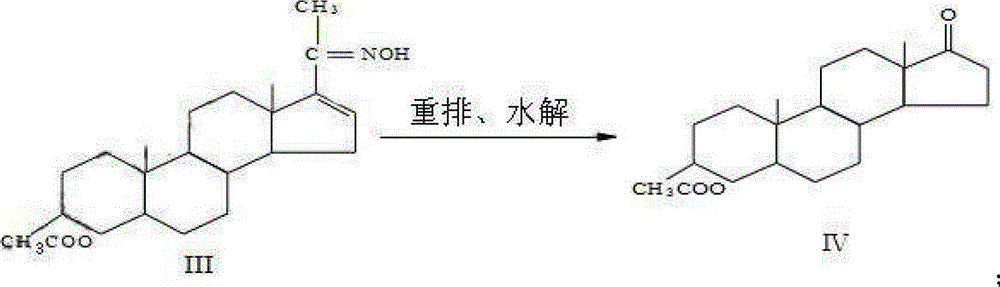
[0031] 2) Preparation of rearrangement IV
[0032] Add 600ml of dehydrated pure benzene and 75g of ketoxime III to the reaction bottle in turn, stir at room temperature to dissolve, then cool down to 5°C, slowly add phosphorus oxychloride-pure benzene mixture dropwise (37.5ml phosphorus oxychloride-75ml) , The temperature of the dropping process is controlled at 5-10°C, and the dropping is completed in 2 hours. Control the temperature at 10-15°C and keep it warm for 2 hours, lower the temperature to 5°C, slowly add...
Embodiment 2
[0036] 1) Preparation of Ketoxime III
[0037] Add 600ml of ethanol, 96ml of pyridine, 48g of hydroxylamine hydrochloride, and 160g of monoenolone acetate II to the reaction flask in sequence, heat slowly, and time reflux for 4 hours. After the reflux, the temperature was lowered to 0°C, and the material was separated by suction filtration. The material was washed with ethanol until no liquid flowed out, and then washed with water until neutral, drained, and dried to obtain 158.2 g of ketoxime III.
[0038] 2) Preparation of rearrangement IV
[0039] Add 1200ml of dehydrated pure benzene and 150g of ketoxime III to the reaction bottle in turn, stir at room temperature to dissolve, then cool down to 5°C, slowly add phosphorus oxychloride-pure benzene mixture (75ml phosphorus oxychloride-150ml pure benzene ), the temperature of the dropping process should be controlled at 5-10°C, and the dropping should be completed in 2 hours. Control the temperature at 10-15°C, hold the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com