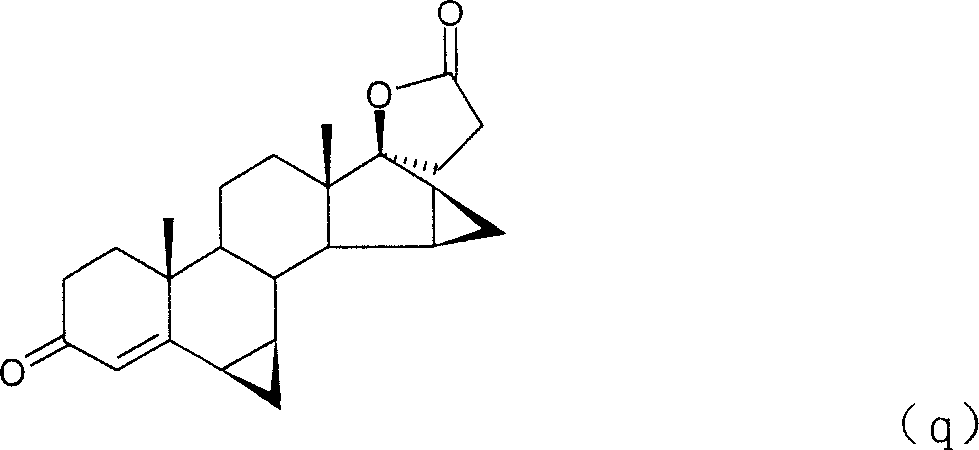
Method for preparing drospirenone intermediate 3beta,5-dihydroxy-15beta,16beta-methylene-5beta-androst-6-en-17-one
A technology of methylene androster and hydroxy androster, applied in the field of medicinal chemistry, can solve the problems of environmental pollution, high equipment investment cost and high technical difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、3
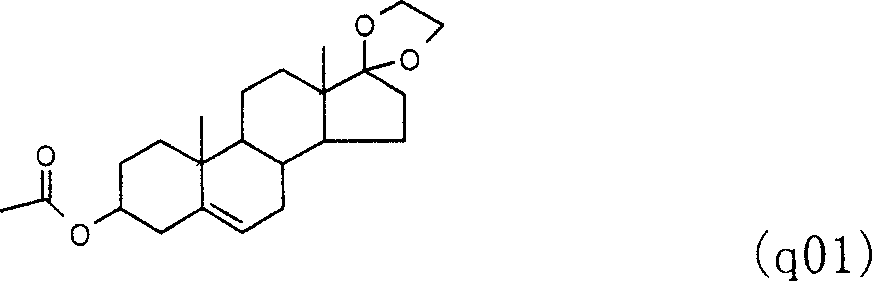
[0072] The preparation of embodiment 1,3β-acetoxy-17-ethylenedioxy-androst-5-ene (q01)
[0073] Add 62.5g of dehydroepiandrosterone acetate, 35.75ml of ethylene glycol, 0.125g of p-toluenesulfonic acid, and 90.0ml of triethyl orthoformate into a four-neck flask, heat to 90°C and reflux for about 1 hour Afterwards, dot plate tracking to determine the end point of the reaction. The solvent was distilled off until the temperature rose to 110°C. The hot solution was poured into 700 ml of methanol preheated and added with 10 ml of pyridine, and stirred for 0.5 hours. Add 200ml of water, cool down to room temperature, suction filter, wash with water, and dry to obtain 69.0g of 3β-acetoxy-17-ethylenedioxyandrost-5-ene. The melting point is 140-142°C.
Embodiment 2、3
[0074] Example 2, Preparation of 3β-acetoxy-16α-bromo-17-ethylenedioxyandrost-5-ene (q02)
[0075] In a four-necked reaction flask, add 112.5ml of tetrahydrofuran, add 37.5g of 3β-acetoxy-17-ethylenedioxyandrost-5-ene (q01), stir and dissolve, add 75g of perbromopyridinium salt and 112ml In the solution prepared in tetrahydrofuran, stir the reaction for 2 hours, keeping the reaction temperature at 20°C. Spot the plate to trace the reaction end point, add 225ml of water to dilute, and distill off tetrahydrofuran under reduced pressure. Suction filtration, washing with water, recrystallization with 85% ethanol after drying the filter cake to obtain 41.0 g of 3β-acetoxy-16α-bromo-17-ethylenedioxyandrost-5-ene. Melting point: 176-179°C.
Embodiment 3
[0076] Embodiment 3, the preparation of 3β-hydroxyl-17-ethylenedioxy-androst-5,15-diene (q03)
[0077] Add 240ml of dimethyl sulfoxide and 19.0g of 3β-acetoxy-16α-bromo-17-ethylenedioxyandrost-5-ene (q02) into the four-necked reaction flask, stir and dissolve, then add 11.0g of t- Potassium butoxide was reacted at 40°C for 20 hours under the protection of nitrogen, poured into 2000ml of anhydrous ether, stirred well, separated into layers, separated the organic phase and concentrated to dryness under reduced pressure, and recrystallized with 85% ethanol , to obtain 13.2 g of 3β-hydroxy-17-ethylenedioxyandrosta-5,15-diene. The melting point is 157-162°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com