Method for preparing androstane-1,4,6-triene-3,17-diketone
A technology of triene and androster, which is applied in the field 1, can solve the problems of many impurities, danger, and high cost, and achieve the effects of reducing pollution, having technical and economic advantages, and improving the total conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
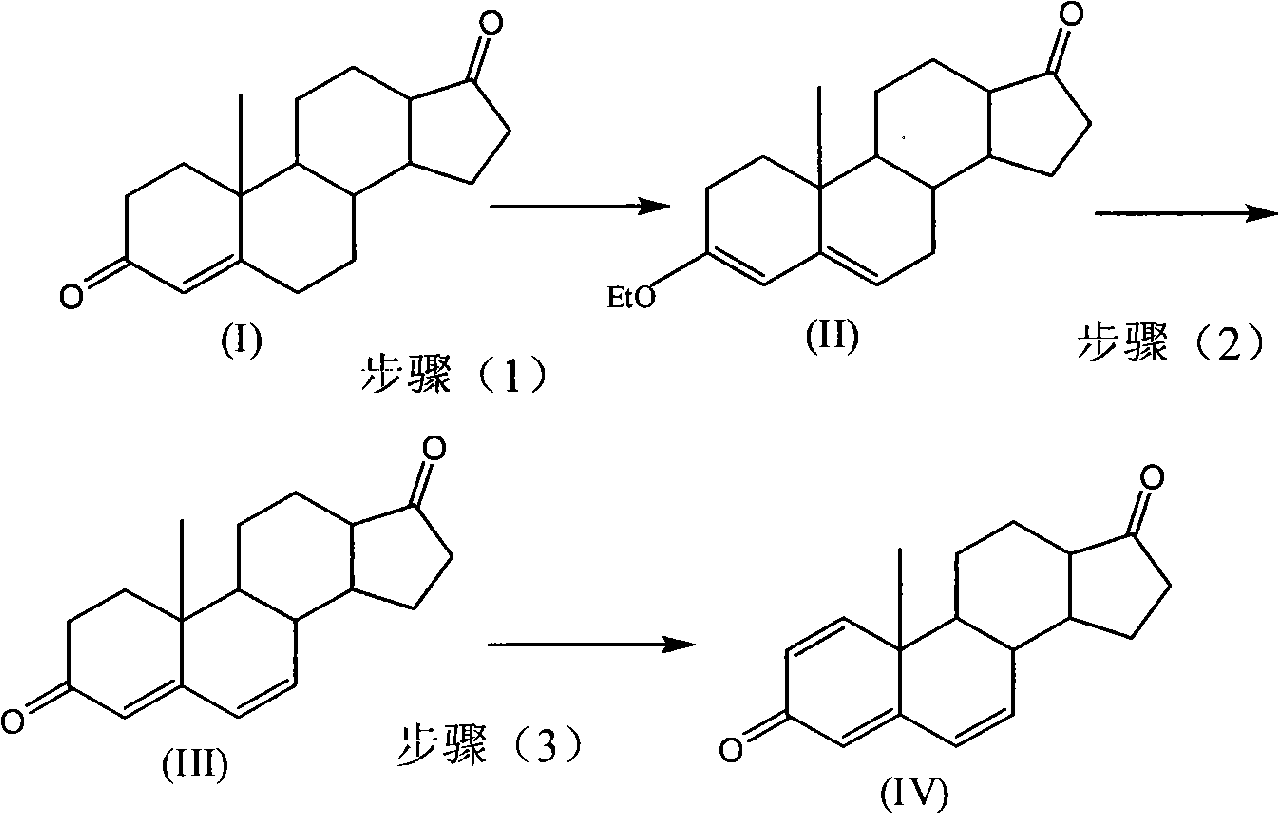
[0029] (1) Get 10g of androst-4-ene 3,17-dione (compound I) into the reaction flask, then add 40ml of absolute ethanol, stir, and pass N 2 , kept at -10°C, added 10ml of triethyl orthoformate, 0.15g of PTS, kept at -10°C for 4 hours until the reaction was complete, added 2ml of pyridine, cooled to -10°C, stood still, filtered, and dried to obtain androster -3-Ethoxy-3,5-dien-17-one 11.0g (Compound II)
[0030] (2) Add the compound (II) obtained in step (1) into the reaction flask, then add 30ml DMF, 1ml water, and pass N 2 Stir, cool down to 0°C, add NBS three times within 30 minutes, 3g each time, add 9g in total, keep at 5-10°C for 2 hours until the reaction is complete, then add Li 2 CO 3 5g, LiBr 2.5g, heat up to 70-80°C, react for 1 hour until the end of the reaction, cool, dilute and filter to obtain 9.8g androst-4,6-diene-3,17-dione (compound III)
[0031] (3) Biological fermentation
[0032]
[0033]
[0034] 300ml seed medium
[0035] Fermentation medium 3...
Embodiment 2
[0042] (1) Get 10g of androst-4-ene 3,17-dione (compound I) into the reaction flask, then add 80ml of methanol, stir, and pass N 2 , kept at 0°C, added 9ml of isopropenyl acetate, 0.08g of PTS, kept at 0°C for 4 hours to complete the reaction, added 1ml of pyridine, added 1000ml of water to dilute, filtered, and dried to obtain androst-3-ethoxy- 3,5-dien-17-one 10.8g (compound II)
[0043] (2) Add the compound (II) obtained in step (1) into the reaction flask, then add 30ml of dioxane, 1ml of water, and pass N 2 Stir, cool down to 10°C, add NBS three times within 30 minutes, 2.4g each time, add 7.2g in total, keep at 0-5°C for 2 hours until the reaction is complete, then add Li 2 CO 3 4g, LiBr 2g, heat up to 50-60°C, react for 1 hour until the end of the reaction, cool, dilute and filter to obtain 9.5g androst-4,6-diene-3,17-dione (Compound III)
[0044] (3) Biological fermentation
[0045]
[0046] 300ml seed medium
[0047] Fermentation medium 300ml
[0048] The above...
Embodiment 3
[0054] (1) Get 10g of androst-4-ene 3,17-dione (compound I) into the reaction flask, then add 50ml of acetone, stir, and pass N 2 , keep at 0°C, add 15ml of triethyl orthoformate, PTS 0.3, keep at 10°C for 4 hours until the reaction is complete, add 4ml of pyridine, add 500ml of water to dilute, let it stand, filter, and dry to obtain androst-3-b Oxy-3,5-dien-17-one 11.1g (compound II)
[0055] (2) Add the compound (II) obtained in step (1) into the reaction flask, then add 60ml DMF, 1ml water, and pass N 2 Stir, cool down to 0°C, add NBS three times within 30 minutes, 3g each time, add 9g in total, keep at 10-15°C for 2 hours until the reaction is complete, then add Li 2 CO 3 5g, LiBr 2.5g, heat up to 60-70°C, react for 1 hour until the end of the reaction, cool, dilute and filter to obtain 9.9g androst-4,6-diene-3,17-dione (compound III)
[0056] (3) Biological fermentation
[0057]
[0058] 300ml seed medium
[0059] Fermentation medium 300ml
[0060] The above pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com