Preparation method and application of 1alpha-dehydroepiandrosterone
A technology for dehydroepiandrosterone and epiandrosterone, which is applied in the directions of steroids, organic chemistry, etc., can solve the problems of long production cycle, high production cost, complicated process, etc., and achieves low production cost, short production cycle, and high technology. Simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
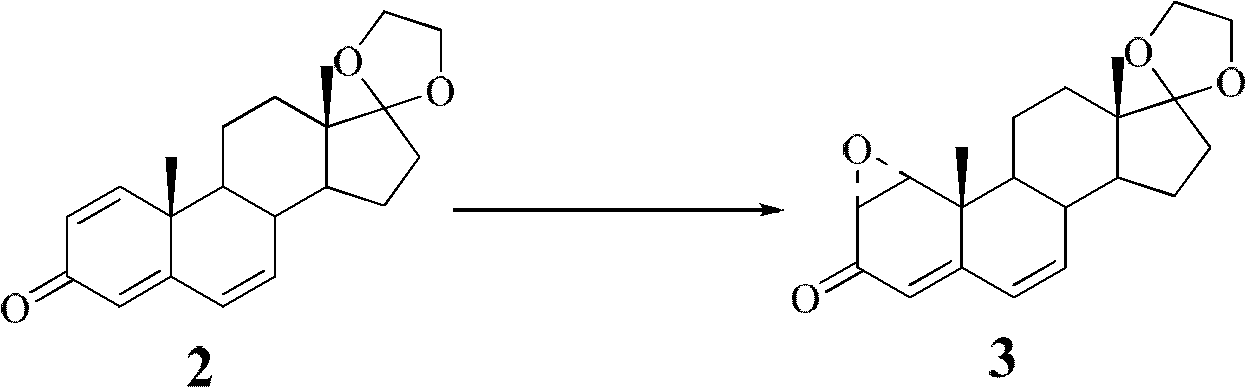
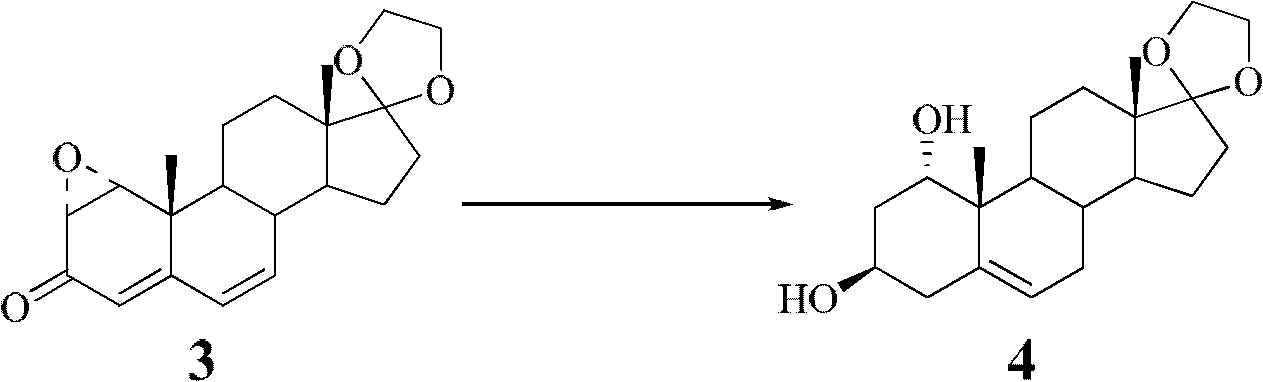
[0028] Preparation of 1α-hydroxy dehydroepiandrosterone according to the preparation method of the present invention
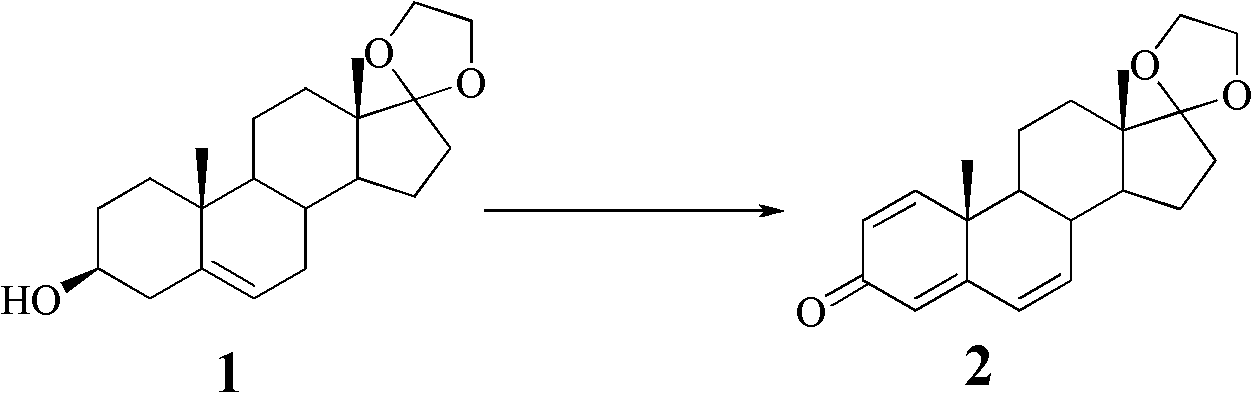
[0029] a. Dehydrogenation and oxidation to prepare intermediate compound II:
[0030] Dissolve 56.5 g of allyl diethyl phosphate and 750 mg of palladium acetate in 100 ml of dimethylformamide, stir at room temperature for 30 min, add 14.5 g of ethylene glycol dehydroepiandrosterone, 37.0 g of sodium carbonate, and , Stirring and reflux for 8h. After adding 10% citric acid, extraction was performed with dichloromethane. After concentration, it was recrystallized with methanol to obtain 12.1 g of intermediate compound II, namely 17,17-ethylenedioxy-1,4,6-triene-androst-3-one, with a yield of 85.0%.
[0031] Melting point: 130~132℃, [α] D : -39 (c=0.55, methanol). 1H-NMR (600MHZ, CDCl 3 )δ: 7.07 (1H, d, J = 10.2Hz), 6.22-6.26 (2H, m), 6.05 (1H, dd, J = 10.2, 1.8Hz), 6.03 (1H, s), 3.82-3.90 (4H , m), 2.28 (1H, t, J=10.2Hz), 2.04 (1H, m), 1.82-1.87 (3H, m), 1...
Embodiment 2
[0041] According to the method and steps of Example 1, the only difference is that the same reaction is carried out with dimethyl allyl phosphate instead of diethyl allyl phosphate, and the yield of step a is 78.6%.
Embodiment 3
[0043]According to the method and steps of Example 1, the only difference is that potassium carbonate is used instead of sodium carbonate to carry out the same reaction, and the yield of step a is 71.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com