Novel method for preparing dehydroepiandrosterone
A new type of dehydroepiandrosterone technology, applied in the field of preparation of dehydroepiandrosterone, can solve the impact on the quality and yield of dehydroepiandrosterone, the poor stability of intermediate 5-AD, and the influence of 5-AD purity Larger problems, etc., to achieve the effect of high industrial application value, single organic solvent, and low requirements for reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation and stability test of embodiment 1 compound II, specifically as follows:
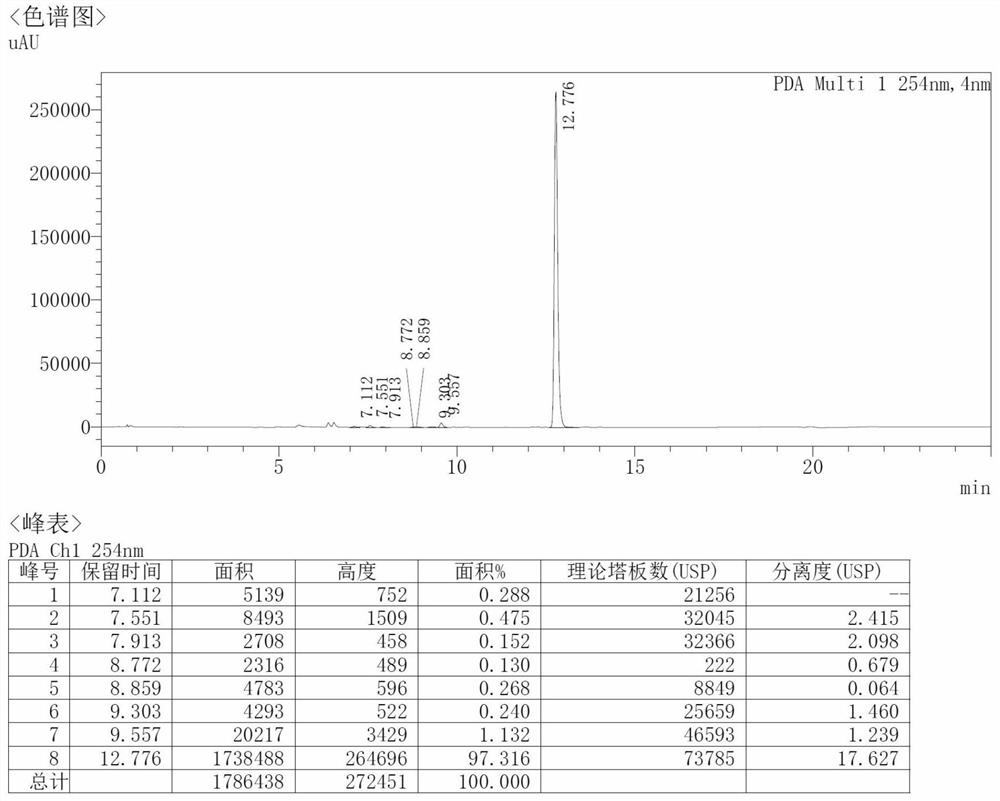
[0043] 1) Under the protection of nitrogen, mix 50g of compound I, 250ml of acetic anhydride and 10g of p-toluenesulfonic acid into the reactor, control the temperature at 20°C and stir the reaction until the reaction of the raw materials is detected by thin layer chromatography, water analysis, filtration, and drying , to obtain 55.6g of compound II, with an HPLC content of 97.3%, such as figure 1 shown.
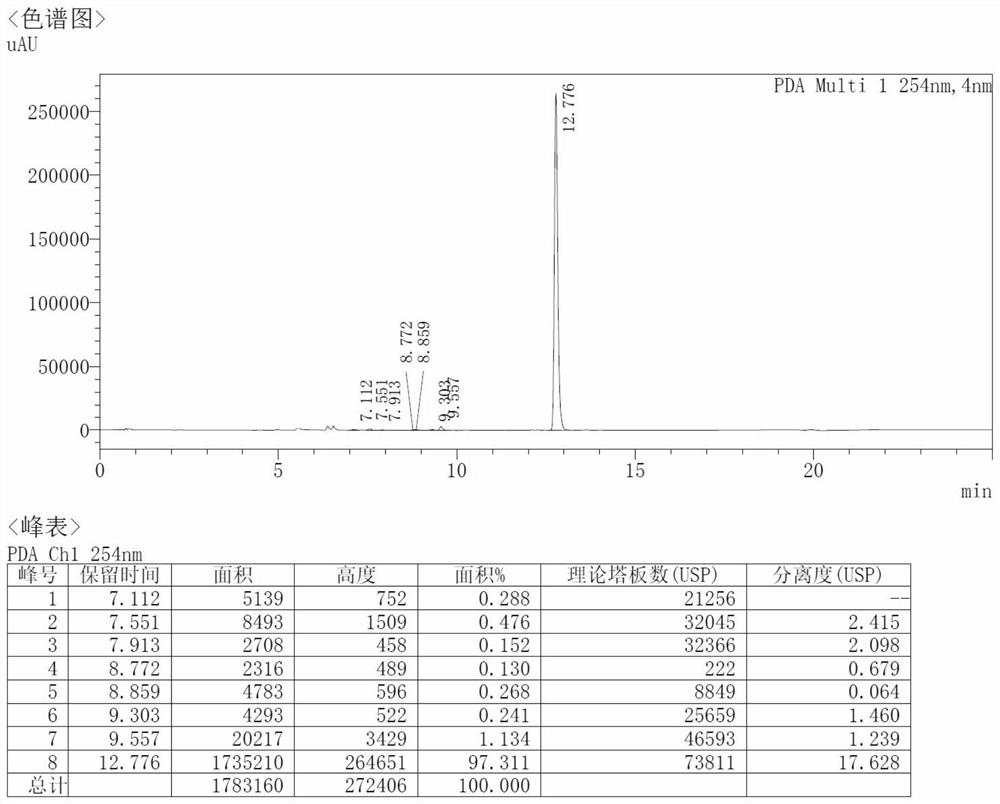
[0044] After placing the above compound II at 20-30°C for 6 hours in a sealed and light-shielded place, the HPLC content was stable at 97.3%, as shown in figure 2 shown.
Embodiment 2
[0045] Embodiment 2 A kind of preparation method of dehydroepiandrosterone, comprises the steps:
[0046] 1) Under the protection of nitrogen, mix 50g of compound I, 250ml of acetic anhydride and 10g of p-toluenesulfonic acid into the reactor, control the temperature at 20°C and stir the reaction until the reaction of the raw materials is detected by thin layer chromatography, water analysis, filtration, and drying 55.5 g of compound II was obtained, with an HPLC content of 97.5%.
[0047] 2) Dissolve 55.5 g of the above-mentioned compound II in 167 ml of tert-butanol, add 390 ml of 100 mM pH6.5 phosphate enzyme buffer solution, start stirring, then add 555 mg of ketoreductase, 277.5 mg of ester hydrolase, and 220 mg of nicotinamide adenine Dinucleotide, 30g glucose, 220mg glucose dehydrogenase, biological enzyme reaction at 26-28°C, and 10% sodium carbonate aqueous solution to control the pH value of 6.8-7.2. After 5 hours, take a sample and test, the conversion rate is 99.3 %...
Embodiment 3
[0048] Embodiment 3 A kind of preparation method of dehydroepiandrosterone, comprises the steps:
[0049] 1) Under nitrogen protection, mix 50 g of compound I, 100 ml of dichloromethane, 150 ml of acetic anhydride, and 25 g of methanesulfonic acid into the reactor, and stir the reaction at 0°C until the reaction of the raw materials is detected by thin-layer chromatography. Concentrate Remove dichloromethane, analyze with water, filter, and dry to obtain 55.6 g of compound II with an HPLC content of 97.6%.
[0050] 2) Dissolve 55.6g of the above-mentioned compound II in 167ml of tert-butanol, add 390ml of 100mM pH6.5 phosphate buffer solution, start stirring, add 834mg of ketoreductase, 417mg of ester hydrolase, and 220mg of nicotinamide adenine dinuclear Nucleic acid, 30g glucose, 220mg glucose dehydrogenase, under the temperature condition of 28-30 ℃, carry out biological enzyme reaction, and use 10% sodium carbonate aqueous solution to control the pH value of 7.2-7.6. Sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com