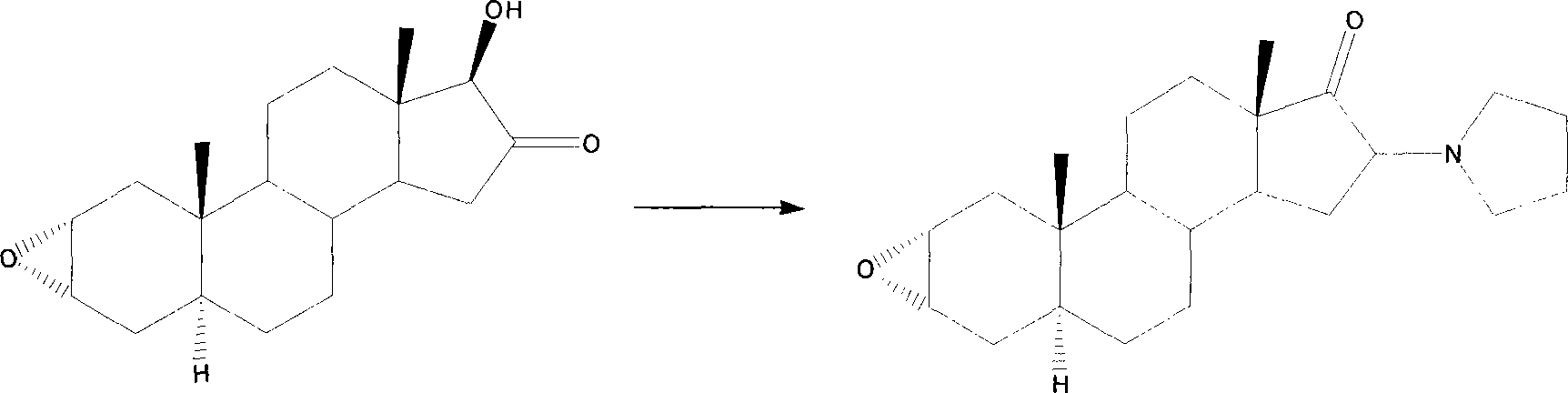
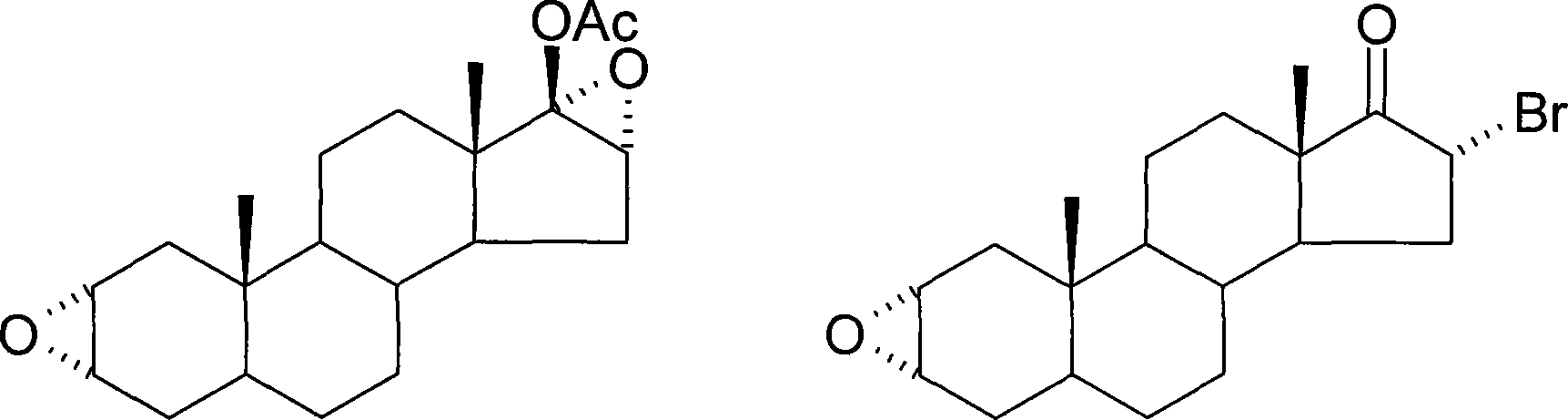
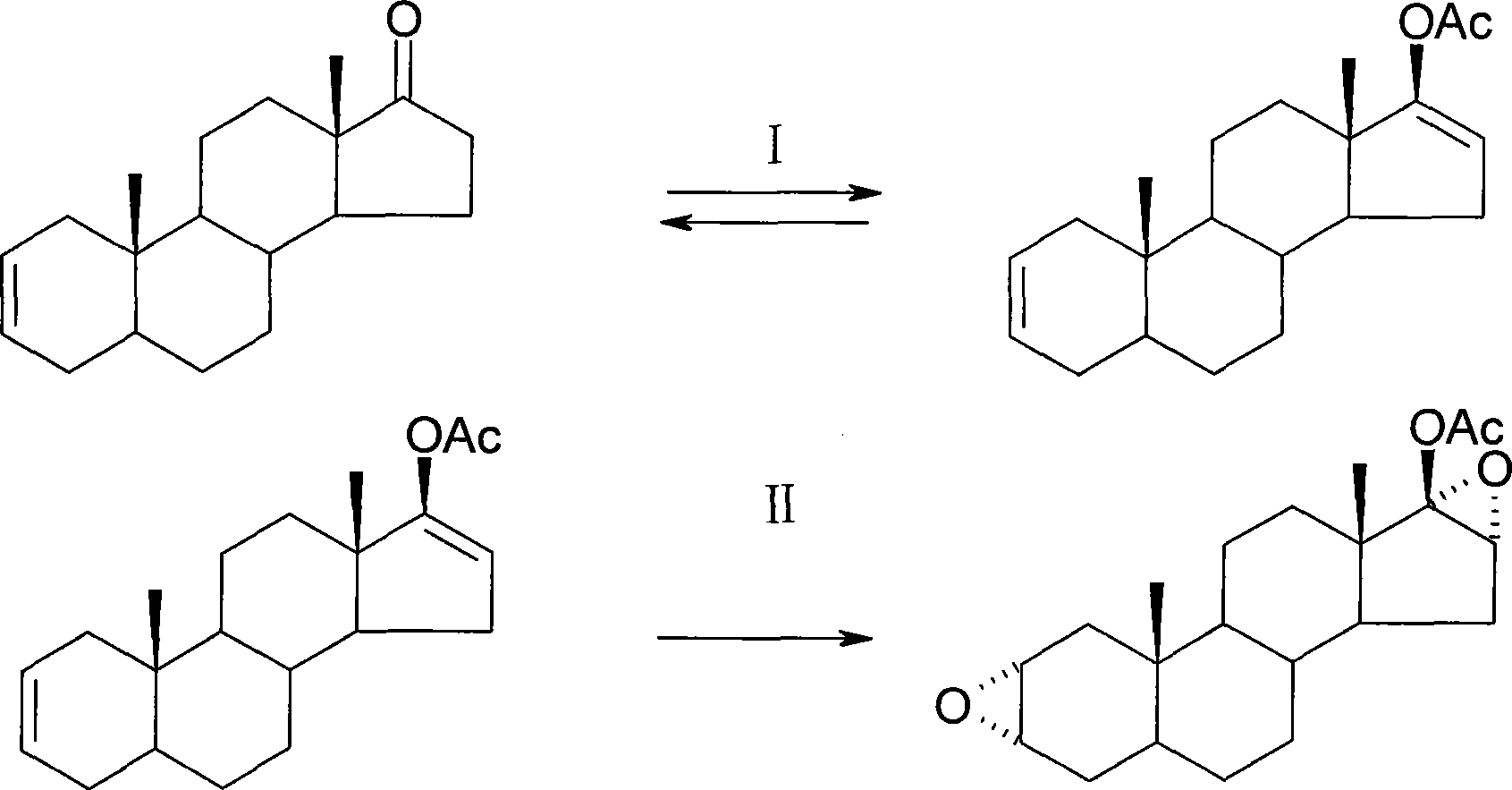
Method of synthesizing 2alpha,3alpha-epoxy-16alpha-bromo-5alpha-androsterone-17-one
A synthetic method and the technology of androsteroids, which are applied in the field of preparation of steroidal muscle relaxant intermediates, can solve problems such as expensive, unstable products, and poor yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] In order to increase the single-step reaction yield, reduce the purification process and losses of each step, and realize the high-yield and high-efficiency industrial preparation requirements, the present invention takes the synthetic route reported in the patent US6090957 as a reference, and has made a great breakthrough in the synthetic process. Table Starting from androsterone, it only needs to go through three steps of reaction, and each step can be directly fed without purification, so as to prepare the important intermediate compound two. Compared with the original method, the synthesis steps of this method are reduced, the operation is simple, the total yield is greatly increased, and the method is economically feasible. The method is as follows:
[0030] 1. Elimination, under the action of p-toluenesulfonic acid-silica gel catalyst, with epiandrosterone (a) as raw material, dehydration in benzene generates 5α-androst-2-en-17-one (c);
[0031] 2. Bromo, 5α-andr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com