Process for preparing acetylacetone by conversion of isopropenyl acetate and special-purpose equipment thereof
A technology of isopropenyl acetate and acetylacetone, which is applied in the field of preparation of acetylacetone, can solve problems such as limitations, and achieve the effects of thin boundary retention layer, high furnace temperature requirements, and reduced carbon deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
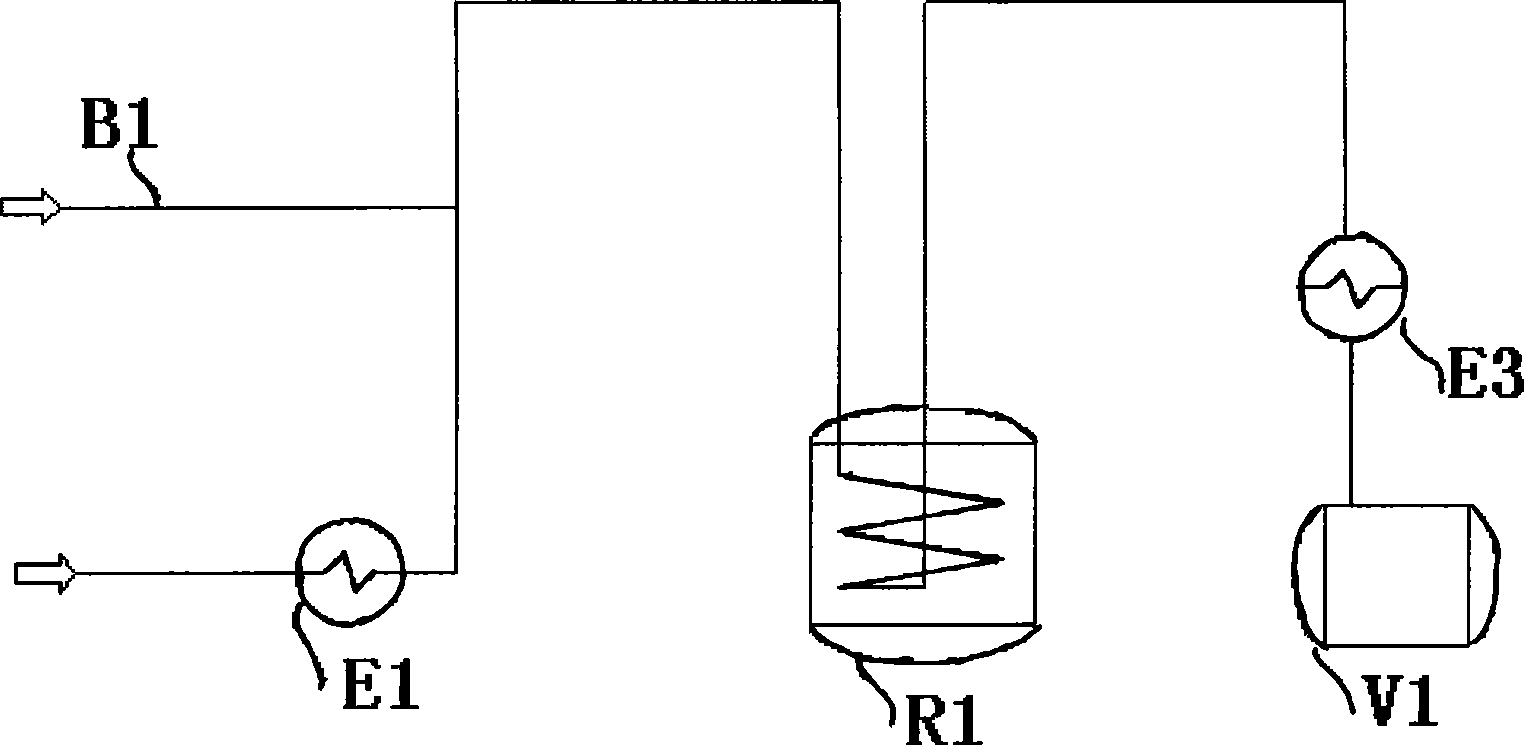
[0038] Embodiment 1 Process one
[0039] The feed rate of isopropenyl acetate is 0.15t / h, the temperature of the vaporizer is 110°C, nitrogen is added as the carrier gas, and the flow rate of nitrogen is 8Nm 3 / h, without using the self-heat exchanger, enter the reformer, the temperature of the reformer is 540°C, the residence time is 2.7s, and then condensed to room temperature through the condenser.
[0040] After analysis, the conversion liquid has a selectivity of 86.2%, a conversion rate of 90.3%, and a yield of 77.8%. The conversion liquid is clear and does not contain carbon particles. By adjusting the nitrogen flow rate, the residence time is further optimized, and the reaction selectivity and yield are further improved.
Embodiment 2
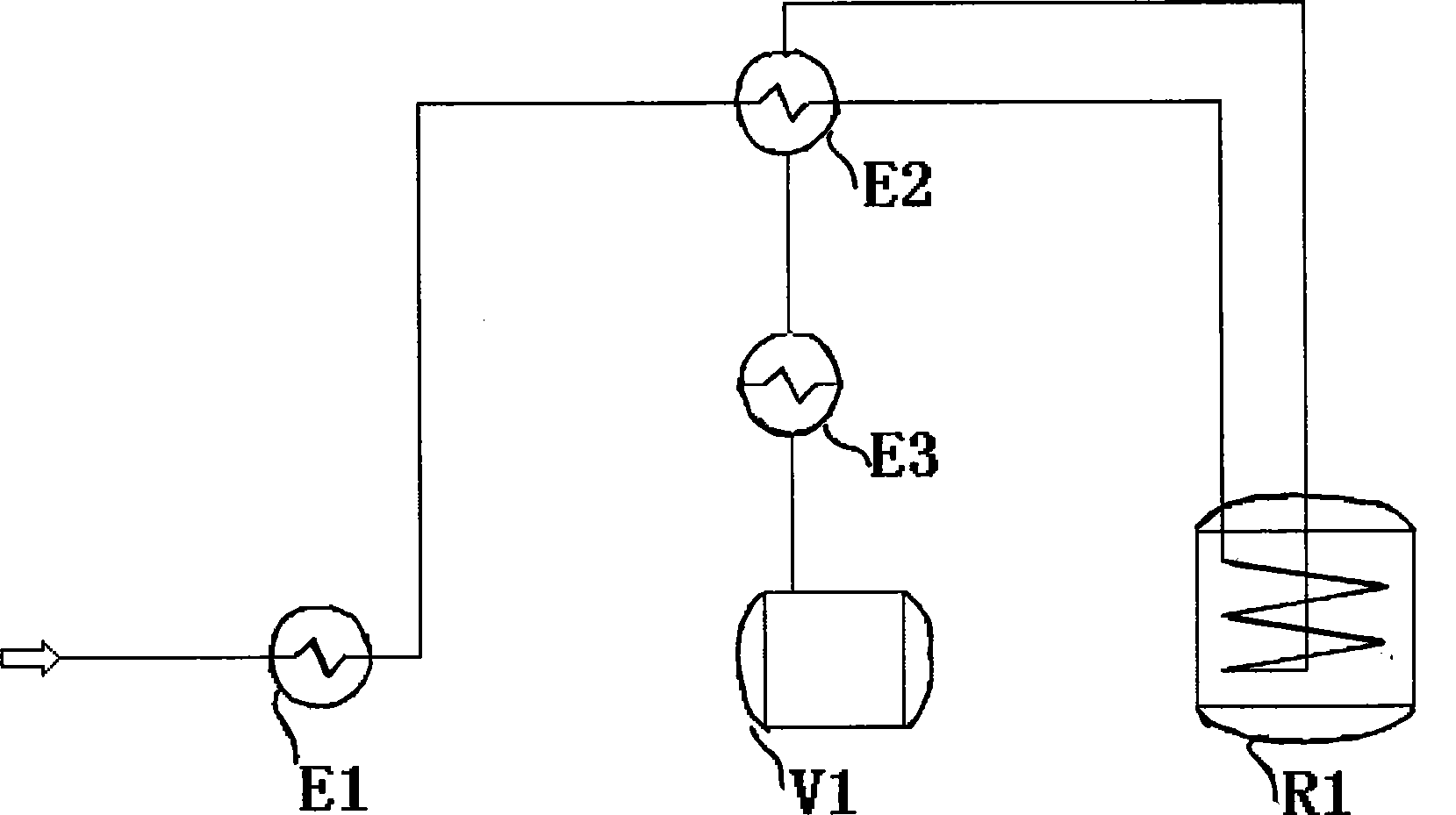
[0041] Embodiment 2 Process two
[0042] The feed rate of isopropenyl acetate is 0.15t / h, the temperature of the vaporizer is 110°C, no gas is loaded, a self-heat exchanger is used, and the preheating temperature at the outlet of the self-heat exchanger is 300°C, and it enters the reformer, and the temperature of the reformer is 580°C. The residence time is 2.7s. After conversion, it is condensed to 320°C through the heat exchanger, and then further condensed to room temperature of 25°C.
[0043] After analysis, the conversion liquid has a selectivity of 81.8%, a conversion rate of 95.5%, and a yield of 78.1%. The conversion liquid contains solid black particles, which are carbon particles. By precisely controlling the temperature of the transformation in stages, the selectivity and yield of the transformation are improved, and at the same time, the waste heat of the transformation gas is reused. Embodiment 3 Process three
Embodiment 3
[0043] After analysis, the conversion liquid has a selectivity of 81.8%, a conversion rate of 95.5%, and a yield of 78.1%. The conversion liquid contains solid black particles, which are carbon particles. By precisely controlling the temperature of the transformation in stages, the selectivity and yield of the transformation are improved, and at the same time, the waste heat of the transformation gas is reused. Embodiment 3 Process three
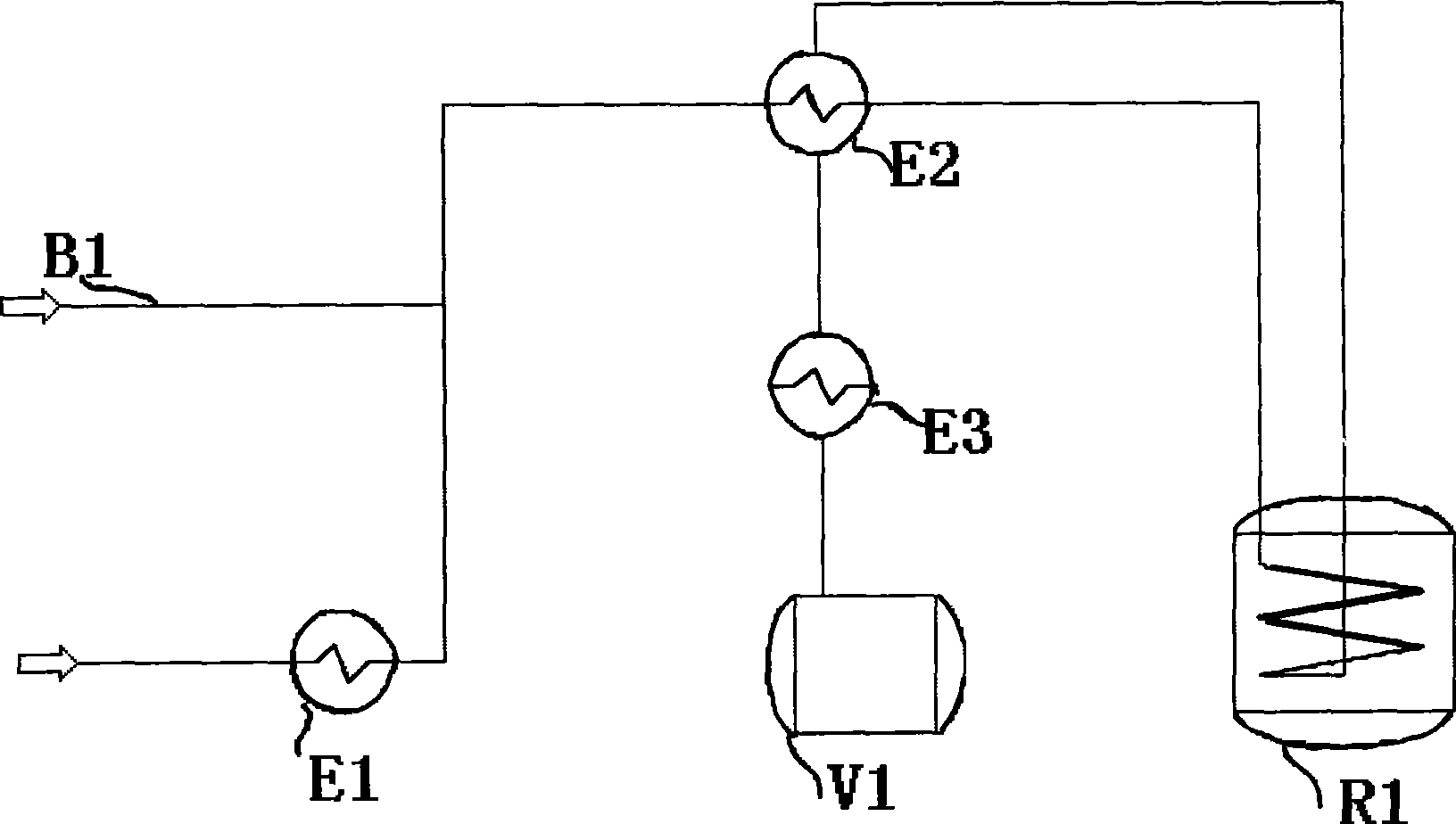
[0044] The feed rate of isopropenyl acetate is 0.15t / h, the temperature of the vaporizer is 110°C, nitrogen is added as the carrier gas, and the flow rate of nitrogen is 8Nm 3 / h, feed from the heat exchanger, the preheating temperature at the outlet of the heat exchanger is 300°C, enter the reformer, the temperature of the reformer is 530°C, the residence time is 2.7s, after the transformation, it is condensed to 320°C, and then further condensed to 20°C.
[0045] After analysis, the conversion liquid has a selectivity of 90.6%, a conversi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com