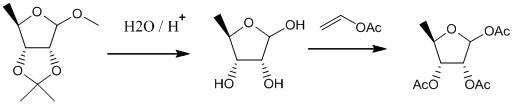
Preparation method of 1,2,3-tri-O-acetyl-5-deoxidization-beta-D-ribose
An acetyl and ribose technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of many by-products, low purity, long operation time, etc., and achieve simple and moderate conditions and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0026] The method of the present embodiment has the following steps:
[0027] ①Add 50.3g of 2,3-O-isopropylidene-5-deoxy-D-furanoside to 300mL of 1wt% hydrochloric acid solution, and 2,3-O-isopropylidene Propylene-5-deoxy-D-furanoside undergoes a hydrolysis reaction of 5-deoxy-D-ribose for 4 hours, and TLC (thin layer chromatography) monitors until the raw material point disappears (dichloromethane:methanol=1:1), After the reaction, 297.3 g of an aqueous solution containing 5-deoxy-D-ribose was obtained.
[0028] ② Add 303.0 g of acetylation reagent vinyl acetate to the aqueous solution containing 5-deoxy-D-ribose obtained in step ①, and generate 1,2,3-tri-O-acetyl at a temperature of 15°C to 25°C The acetylation reaction of base-5-deoxy-D-ribose was carried out for 3 hours. During the reaction, 5% NaOH solution was added dropwise to keep the pH at 10. An aqueous solution containing 1,2,3-tri-O-acetyl-5-deoxy-D-ribose was obtained.
[0029]③ After the acetylation reaction, ...
Embodiment 2)
[0034] The method of the present embodiment has the following steps:
[0035] ①Add 51.2g of 2,3-O-isopropylidene-5-deoxy-D-furanoside to 250mL of sulfuric acid solution with a concentration of 1wt%. Propylene-5-deoxy-D-methylfuranoside undergoes a hydrolysis reaction of 5-deoxy-D-ribose for 3 hours, and TLC monitors until the raw material point disappears (dichloromethane:methanol=1:1). After the reaction, the obtained 237.2 g of an aqueous solution containing 5-deoxy-D-ribose.
[0036] ② Add 242.2 g of acetylation reagent vinyl acetate to the aqueous solution containing 5-deoxy-D-ribose obtained in step ①, and generate 1,2,3-tri-O-acetyl at a temperature of 15°C to 25°C The acetylation reaction of base-5-deoxy-D-ribose was 4h, and the concentration of 10% Na was added dropwise during the reaction 3 PO 4 solution to maintain a pH of 10. An aqueous solution containing 1,2,3-tri-O-acetyl-5-deoxy-D-ribose was obtained.
[0037] ③ After the acetylation reaction, carry out pos...
Embodiment 3)
[0042] The method of the present embodiment has the following steps:
[0043] ①Add 50.4g of 2,3-O-isopropylidene-5-deoxy-D-furanoside to 250mL of sulfuric acid solution with a concentration of 1wt%. Propylene-5-deoxy-D-methylfuranoside undergoes a hydrolysis reaction of 5-deoxy-D-ribose for 3 hours, and TLC monitors until the raw material point disappears (dichloromethane:methanol=1:1). After the reaction, the obtained 230.3 g of an aqueous solution containing 5-deoxy-D-ribose.
[0044] ② Add 232.1 g of acetylation reagent isopropenyl acetate to the aqueous solution containing 5-deoxy-D-ribose obtained in step ①, and generate 1,2,3-tri-O- The acetylation reaction of acetyl-5-deoxy-D-ribose was 3h, and the concentration of 10% Na was added dropwise during the reaction 2 CO 3 solution to maintain a pH of 10. An aqueous solution containing 1,2,3-tri-O-acetyl-5-deoxy-D-ribose was obtained.
[0045] ③ After the acetylation reaction, carry out post-treatment to obtain the crude...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com