DBAT mutation enzyme D166H and application thereof
A D166H, mutant enzyme technology, applied in the field of biomolecular catalysis, can solve problems such as enzyme instability and inactivation, and achieve the effects of reducing production cost, improving utilization rate and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Obtaining of wild-type DBAT
[0030] The gene sequence of the DBAT enzyme published on NCBI is SEQ ID NO: 1 (GenBank: JQ029678.1), GGAATTC (EcoRI restriction site) is added before the start codon ATG, and GCGGCCGCTTTA (NotⅠ) is added after the stop codon TGA Restriction site) to obtain the gene sequence of the DBAT enzyme with the restriction site.
[0031] Use restriction endonuclease EcoRI and NotI (Invitrogen Company) to carry out double enzyme digestion to the gene of DBAT enzyme; Simultaneously, use restriction endonuclease EcoRI and NotI to carry out double enzyme digestion to pET-32a (+) vector (Invitrogen Company) . The digested product was purified using a gel purification kit, and the above two digested products were ligated with T4 DNA ligase (Invitrogen). The ligation product was transformed into E.coliDH5α competent cells (Invitrogen Company), a small amount of plasmid extraction, enzyme digestion identification, PCR (primers are dbat-F and dbat...
Embodiment 2DB
[0034] The acquisition of embodiment 2DBAT mutant enzyme
[0035] (1) Construction of mutant enzyme vectors by using the whole plasmid PCR method
[0036] 1. A small amount of plasmid extraction of the recombinant plasmid pET-DBAT;
[0037] 2. Using the plasmid pET-DBAT as a template, combined with the mutation primers at both ends, using overlap extension PCR technology, amplified the dbat plasmid containing the mutation site. The PCR system is as follows:
[0038]
[0039] The Primer-F and Primer-R need to be changed according to different mutant enzymes, as shown in Table 1.
[0040] The primer pair D166AF and D166AR are used to obtain the DBAT mutant enzyme D166A, the amino acid sequence of which is SEQ ID NO: 4, and the coding nucleotide sequence is SEQ ID NO: 3.
[0041] The primer pair D166HF and D166HR are used to obtain DBAT mutant enzyme D166H or D166HR363H; the amino acid sequence of DBAT mutant enzyme D166H is SEQ ID NO: 6, and the encoding nucleotide sequence...
Embodiment 3
[0078] Example 3 Fermentation verification and enzyme activity analysis of mutant enzyme engineering strain TB21-D166H
[0079] (1) Fermentation, protein induction and purification of engineering strains
[0080] 1. Protein induced expression
[0081] The expression host E.coliBL21 was transformed with the recombinant plasmid to obtain the recombinant expression strain TBL21-D166H. Inoculate the positive clone into 100 mL of LB liquid medium (with 100 μg / mL of ampicillin added) according to the inoculum amount of 1%, cultivate at 37°C and 220 rpm until OD600=0.6-0.8, and add the final concentration of 1 mM inducer IPTG, 28 ℃ induction 3h.
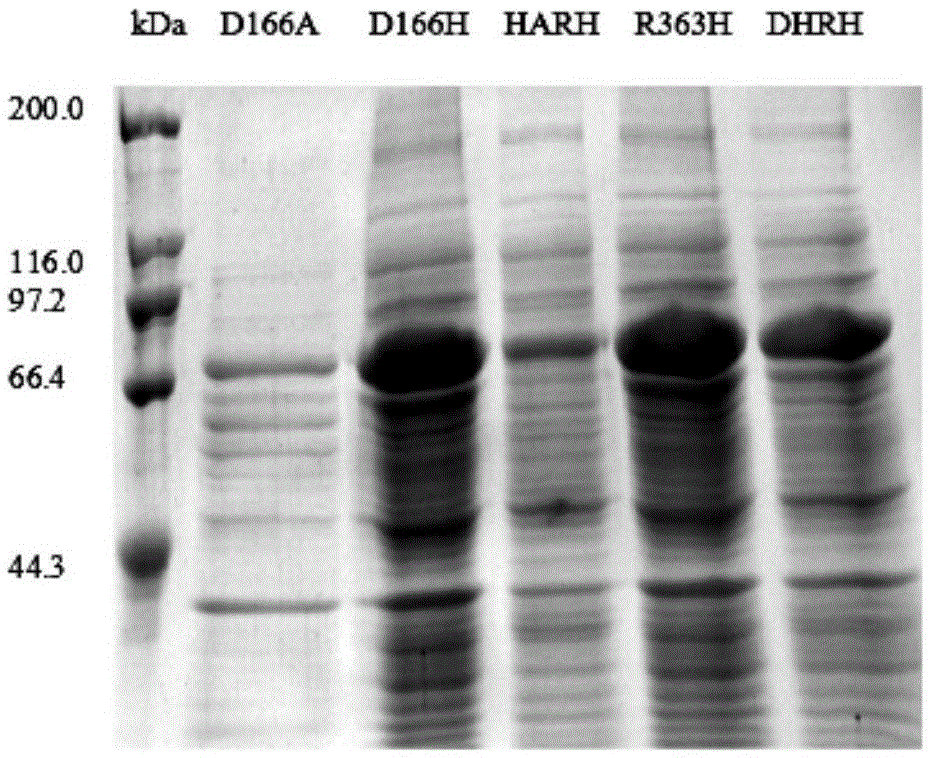
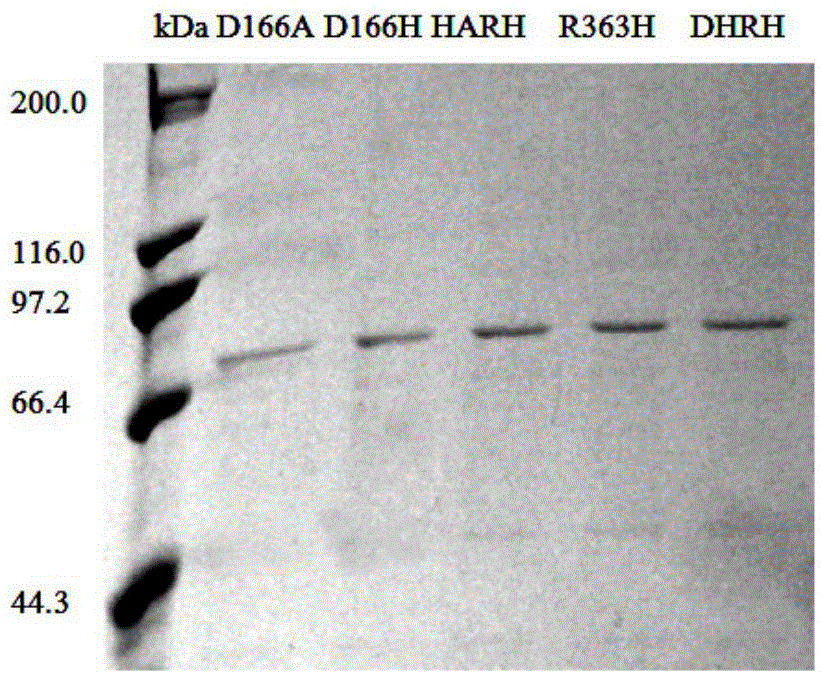
[0082]The cells were collected by centrifugation at 8000 rpm for 10 min at 4°C, and resuspended by adding 20 mL of phosphate buffer (PBS, pH 7.4) per 100 mL of the original fermentation broth. The resuspended bacteria were crushed with an ultrasonic cell disruptor. The total ultrasonic time was 12 min, the working time was 6 s, the inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com