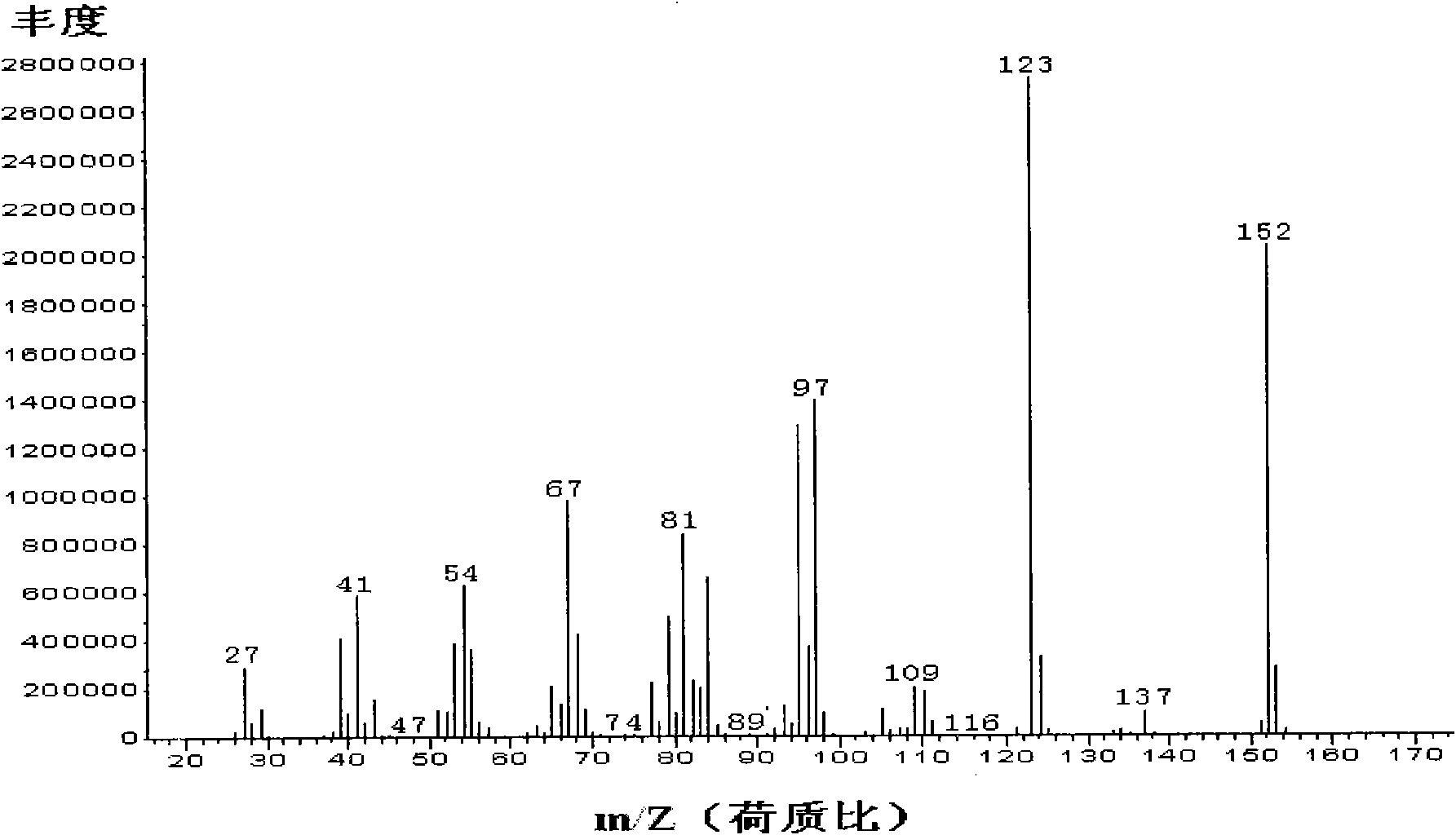
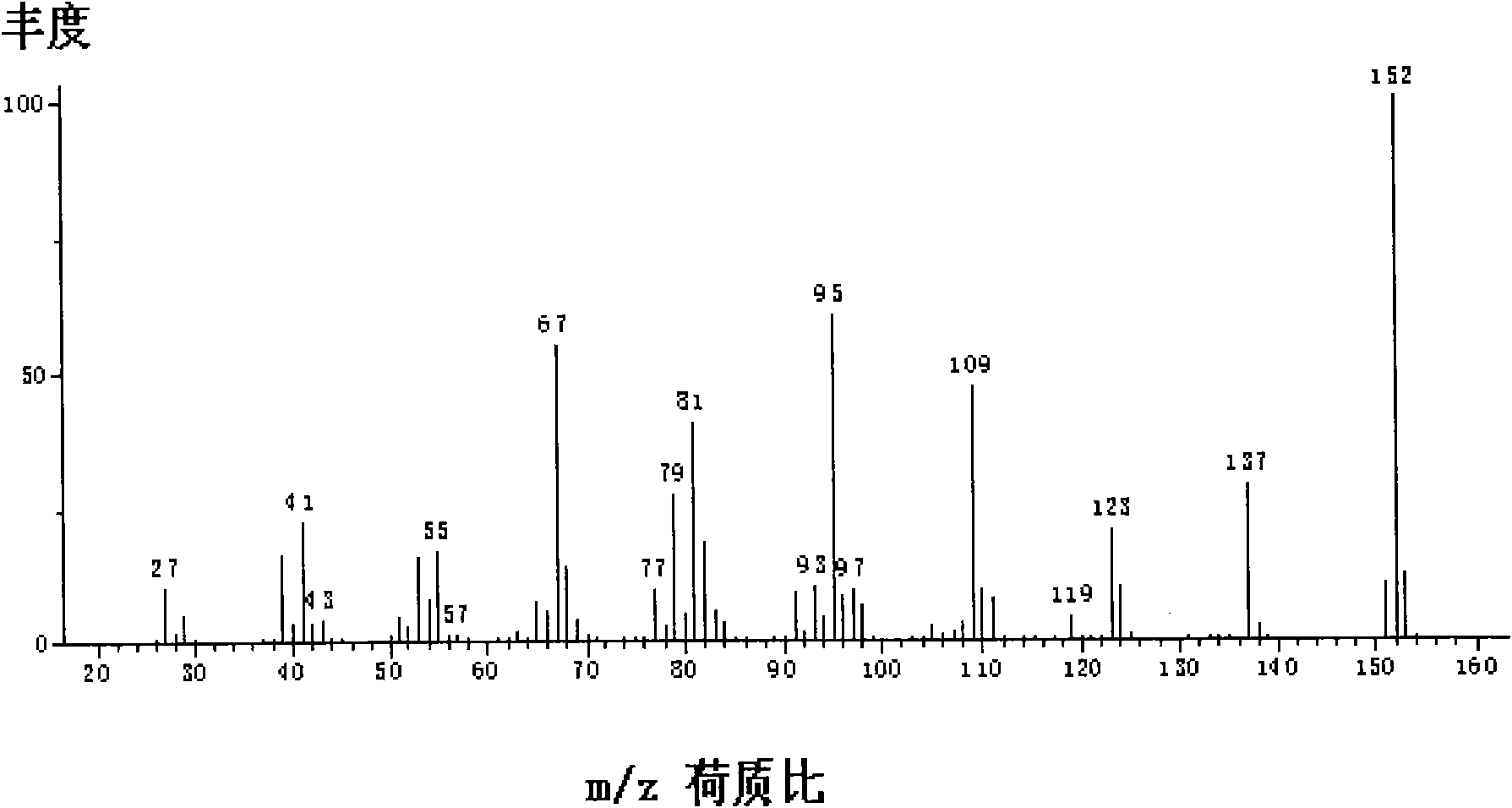
Improved method for producing 2-alkylene alicyclic ketone
A technology for alkylene esters and alicyclic ketones, which is applied in the field of environmentally friendly production of 2-alkylene alicyclic ketones, can solve the problems of slow production speed and easy self-polymerization of fatty aldehydes, and achieve increased production speed and reduced side effects. product, fully utilized effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]Embodiment 1 A kind of method adopting clean technology to synthesize 2-alkylene cycloaliphatic ketones comprises the following steps:
[0042] (1) Put a certain amount of alicyclic ketones, fatty aldehydes, weakly basic ion exchange resins, acids (chemical compounds) and aqueous solutions containing metal ions into the reactor at one time and mix them at room temperature, then heat up to 30°C. Continue to react until the basic reaction of aliphatic aldehyde is complete;
[0043] (2) separate resin, water layer (phase) and oily phase (organic phase) from the reaction mixture that step (1) obtains, for the resin that still has reactivity, can be used for next batch of reaction use, the resin that loses activity sends to regenerate; the water phase can be recycled;
[0044] (3) the oil phase (organic phase) that step (2) obtains adopts vacuum distillation to remove the light fraction containing unreacted cycloaliphatic ketone and some low boiling point substances, and thi...
Embodiment 2
[0046] Embodiment 2 A kind of method adopting clean technology to synthesize 2-alkylene alicyclic ketones comprises the following steps:
[0047] (1) First put alicyclic ketone, weakly basic ion exchange resin, acid (active compound) and aqueous solution containing metal ions into the reactor at one time, after heating up to 50°C, add aliphatic aldehyde dropwise under stirring, and then maintain Continue to react at this temperature until the basic reaction of aliphatic aldehyde is complete;
[0048] (2) separate resin, water layer (phase) and oily phase (organic phase) from the reaction mixture that step (1) obtains, for the resin that still has reactivity, can be used for next batch of reaction use, the resin that loses activity sends to regenerate; the water phase can be recycled;
[0049] (3) the oil phase (organic phase) that step (2) obtains adopts vacuum distillation to remove the light fraction containing unreacted cycloaliphatic ketones and low boilers, and the light...
Embodiment 3
[0051] Embodiment 3 A kind of method adopting clean technology to synthesize 2-alkylene alicyclic ketones comprises the following steps:
[0052] (1) Put a certain amount of alicyclic ketones and aliphatic aldehydes into the reactor, heat up to 70°C, and then put in a mixture of weakly basic ion exchange resins, acids (active compounds) and aqueous solutions containing metal ions, and then maintain this temperature Continue to react until the basic reaction of fatty aldehyde is complete;
[0053] (2) separate resin, water layer (phase) and oily phase (organic phase) from the reaction mixture that step (1) obtains, for the resin that still has reactivity, can be used for next batch of reaction use, the resin that loses activity sends to regenerate; the water phase can be recycled;
[0054] (3) the oil phase (organic phase) that step (2) obtains adopts vacuum distillation to remove the light fraction containing unreacted cycloaliphatic ketones and low boilers, and the light fra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com