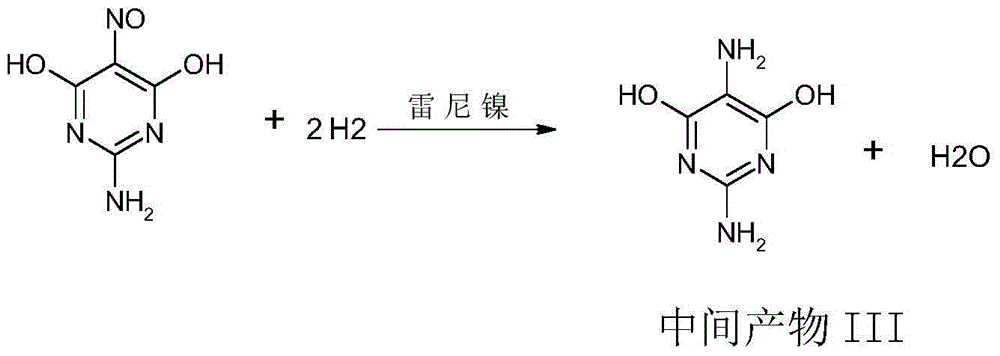
Synthetic method for 2,5-diamino-4,6-dihydroxypyrimidine hydrochloride
A technology of hydroxypyrimidine hydrochloride and synthesis method, which is applied in the field of synthesis of 2,5-diamino-4,6-dihydroxypyrimidine hydrochloride, can solve the problem of high catalyst price, long reaction time, cumbersome post-processing, etc. problem, to achieve the effect of high purity, short reaction time, and complete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
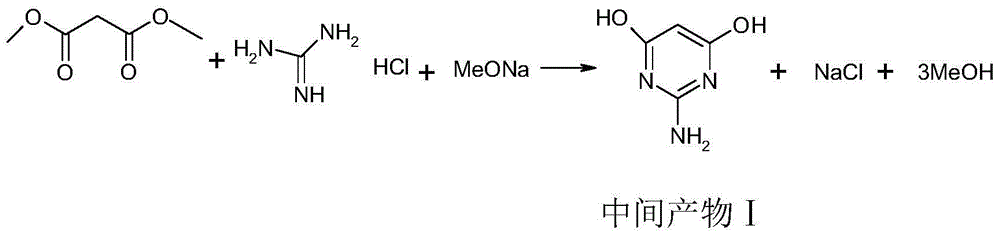
[0023] 1), put 298.7g of 28-30% (mass fraction) sodium methoxide solution and 76.4g of guanidine hydrochloride into a four-necked bottle, stir and heat up to 50°C, add 114.4g of dimethyl malonate dropwise, during the dropwise addition Keep the temperature at 50°C, and keep the reaction at 50°C for 3 hours after the dropwise addition. After the reaction was completed, the methanol was recovered by distillation under reduced pressure, and 400 g of water was added after the recovery, and then 30-35% hydrochloric acid was added to adjust the pH to 3-4, and 165 g of hydrochloric acid was removed in total, and the intermediate product I was obtained after cooling down to room temperature, suction filtration, and drying. 98.2 g of 2-amino-4,6-dihydroxypyrimidine.
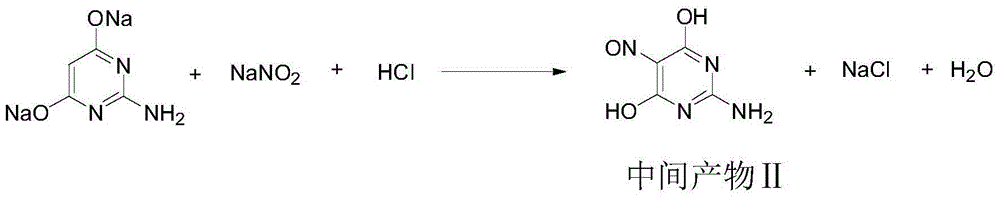
[0024] 2), add intermediate product I98.2g and water 320g in the reactor, then add 30% (mass fraction) sodium hydroxide aqueous solution 208g, stir and heat up to 40 ℃, add sodium nitrite 63.5g, then start to drop 30- 35%...
Embodiment 2
[0028] 1), 373.3g of 28-30% (mass fraction) sodium methoxide solution and 76.4g of guanidine hydrochloride were dropped into a four-necked bottle, stirred and heated to 40°C, and 124.8g of dimethyl malonate was added dropwise. The temperature was 40° C., and the temperature was kept at 40° C. for 5 hours after the dropwise addition was completed. After the reaction was completed, methanol was recovered by distillation under reduced pressure, and 400 g of water was added after the recovery, and then glacial acetic acid was added to adjust the pH to pH=4-5, and 125 g of deglacial acetic acid was shared, and the intermediate product I, 2- Amino-4,6-dihydroxypyrimidine 98.8g.
[0029] 2), add the intermediate product I, 98.8g of 2-amino-4,6-dihydroxypyrimidine and 350g of water into the reactor, then add 195g of 30% (mass fraction) sodium hydroxide aqueous solution, stir and heat up to 50°C, add Sodium nitrite 66.2g, then start to add 30-35% (mass fraction) hydrochloric acid drop...
Embodiment 3
[0033]1), put 448.0g of 28-30% (mass fraction) sodium methoxide solution and 76.4g of guanidine hydrochloride into a four-necked bottle, stir and heat up to 45°C, add 135.2g of dimethyl malonate dropwise, and keep The temperature was 45°C, and the reaction was kept at 45°C for 4 hours after the dropwise addition. After the reaction was completed, methanol was recovered by distillation under reduced pressure, and 400 g of water was added after the recovery, and then glacial acetic acid was added to adjust the pH to pH=4-5, and 126 g of deglacial acetic acid was shared, and the intermediate product I, 2- Amino-4,6-dihydroxypyrimidine 99.3 g.
[0034] 2), add the intermediate product I, 99.3g of 2-amino-4,6-dihydroxypyrimidine and 380g of water into the reactor, then add 198g of 30% (mass fraction) sodium hydroxide aqueous solution, stir and heat up to 50°C, add Sodium nitrite 69.0g, then start to add 30-35% (mass fraction) hydrochloric acid dropwise to adjust to pH = 2-3, after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com