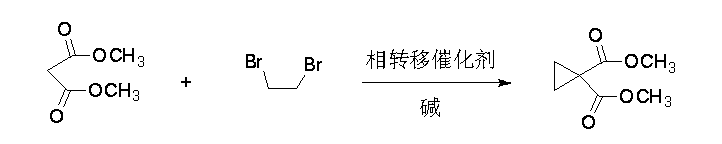
Synthetic process of 1, 1-cyclopropane dicarboxylic acid dimethyl ester
A technology of dimethyl diformate and dimethyl malonate, which is applied in the field of synthesis of pharmaceutical raw material intermediates, can solve the problems of high boiling point and difficult recovery of polyethylene glycol dimethyl ether, and saves reaction time and is easy to operate. , the effect of cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 17.8mL (0.21 mol) dimethyl malonate, 57.2mL (0.66 mol) dibromoethane, 33.1g (0.24 mol) potassium carbonate, 0.26g (0.8 mmol) tetrabutylammonium bromide, 50mL N, N-dimethylformamide joins in the reaction bottle of band condenser and water trap, 120 o C reacted for 3h. After the reaction, dibromoethane and N,N-dimethylformamide were recovered by distillation under reduced pressure, dichloromethane was added to the residue, stirred and filtered, and the filtrate was concentrated to obtain 28.2 g of dimethyl 1,1-cyclopropanedicarboxylate , yield 85%.
Embodiment 2
[0021] 17.8mL (0.21 mol) dimethyl malonate, 54.6mL (0.63 mol) dibromoethane, 29.0g (0.21 mol) potassium carbonate, 0.26g (0.8 mmol) tetrabutylammonium bromide, 50mL N, N-dimethylformamide joins in the reaction bottle of band condenser and water trap, 110 o C reacted for 3h. After the reaction, dibromoethane and N,N-dimethylformamide were recovered by distillation under reduced pressure, dichloromethane was added to the residue, stirred and filtered, and the filtrate was concentrated to obtain 24.9 g of dimethyl 1,1-cyclopropanedicarboxylate , yield 75%.
Embodiment 3
[0023] 17.8mL (0.21 mol) dimethyl malonate, 63.7mL (0.74 mol) dibromoethane, 33.1g (0.24 mol) potassium carbonate, 0.34g (1.1 mmol) tetrabutylammonium bromide, 50mL N, N-dimethylformamide joins in the reaction bottle of band condenser and water trap, 130 o C reacted for 3h. After the reaction, dibromoethane and N,N-dimethylformamide were recovered by distillation under reduced pressure, dichloromethane was added to the residue, stirred and filtered, and the filtrate was concentrated to obtain 28.9 g of dimethyl 1,1-cyclopropanedicarboxylate , yield 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com