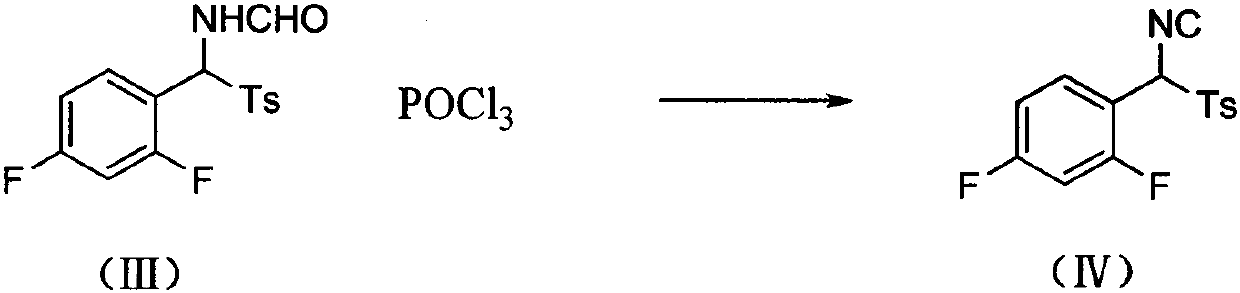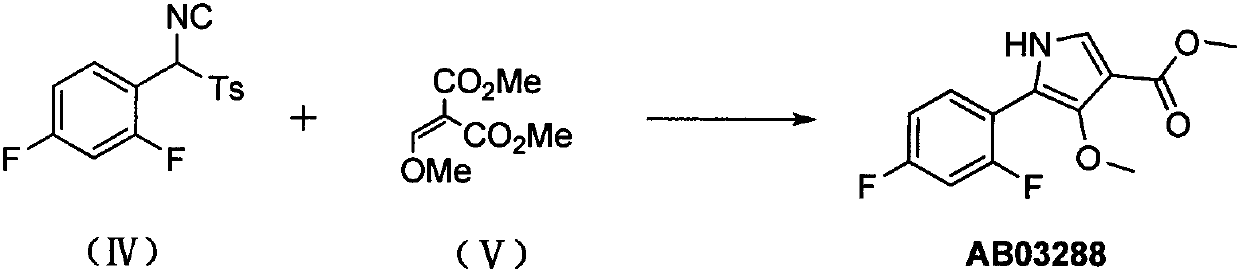Preparation method of 4-methoxyl pyrrole intermediate
A technology of methoxypyrrole and intermediates, which is applied in the field of chemical drug synthesis, can solve the problems of inaccessibility of starting materials, inability to carry out large-scale production, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The following examples can enable those skilled in the art to understand the present invention more comprehensively, but the present invention is not limited to the scope of the described examples.
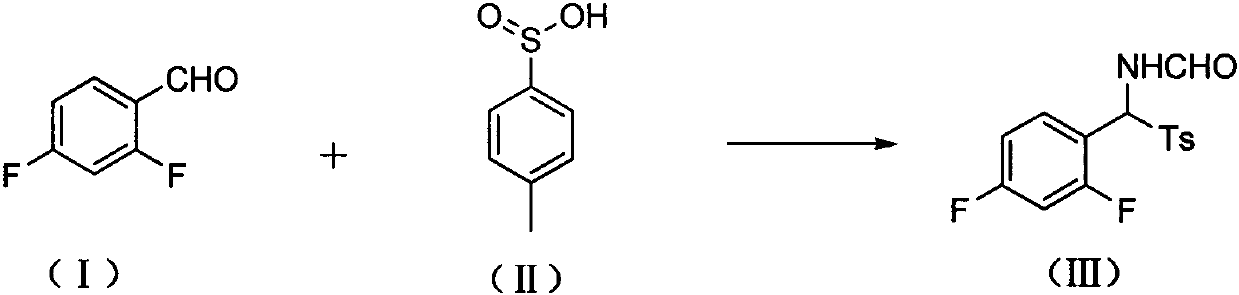
[0027] 1. Synthesis of (III): Get 75g (I), 108g formamide, 3.35g camphorsulfonic acid, and 75g (II) into the reaction flask. Stir in an oil bath under nitrogen protection and raise the temperature to 65-70°C and stir for 16h. Cool to room temperature (15-30°C), add 300ml of water, stir for 30min, and filter. The filter cake was rinsed 3 times with 50ml of water. Scoop out the filter cake. Add 200ml of methanol, stir at room temperature 15-30°C for 30min and then filter. Add 200ml of methanol to the filter cake, stir at room temperature 15-30°C for 30min, and then filter. After the filter cake was dried, 117 g of white powder were obtained. Yield 75%.
[0028] 2. Synthesis of (IV): Dissolve 68.2g of (III), 680ml of anhydrous tetrahydrofuran, and add 64.28g of phosphoru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com