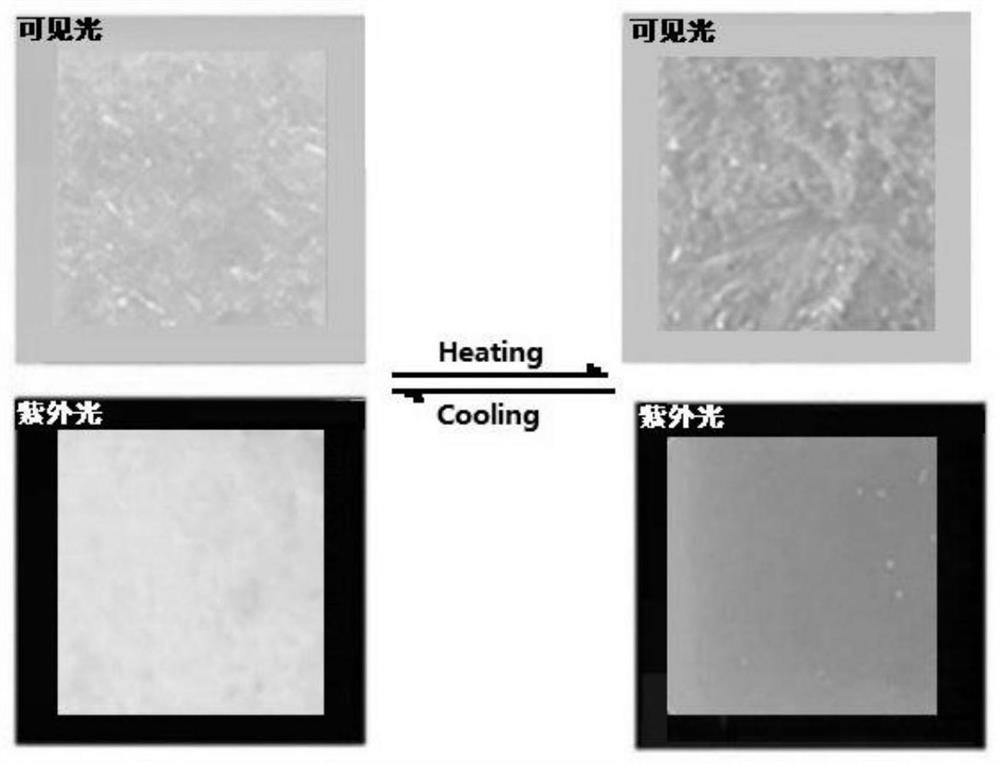
Thermally-stimulated fluorescence/visible light color double-response capsule and synthesis method thereof
A synthesis method and thermal stimulation technology, applied in chemical instruments and methods, luminescent materials, textiles and papermaking, etc., can solve problems such as difficulty in meeting, change in judgment of temperature changes, inapplicability, etc., to simplify the preparation process and maintain stable performance. , The effect of temperature sensitive response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve 10mmol 4-dimethylaminosalicylaldehyde in 5mL ethanol, add 5mmol dimethyl malonate and 0.5mL catalyst piperidine, stir well, and keep stirring the mixture at 80°C for 2h under reflux 50mL of deionized water was added after the reaction, followed by extraction with ethyl acetate 3 times, adding 50mL each time to obtain a crude product, which was then separated and purified by chromatography, and dried to obtain a yellow powder color body molecule.
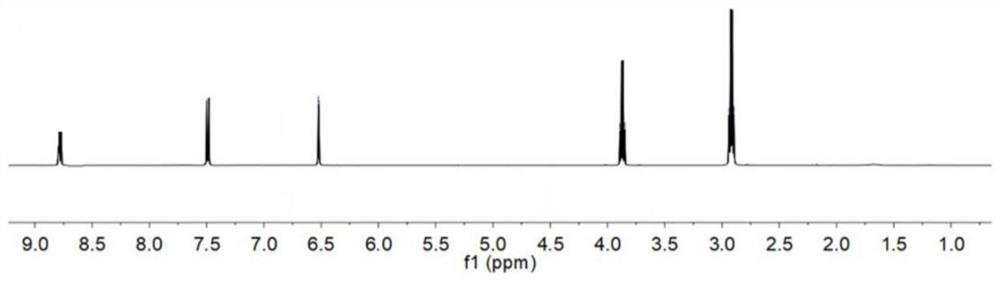
[0029] 1 H NMR test results such as figure 1 As shown, it can be found from the figure that there are significant absorption peaks at chemical shifts 2.75-2.90, 3.7-3.9, and 8.7-8.9, which are characteristic peaks of hydrogen on methyl and vinyl groups, and chemical shifts are 6.5-6.6, 7.5- The absorption peak at 7.6 is the characteristic peak of hydrogen on the benzene ring.
[0030] (2) 0.1 g of the purified product in step (1) was mixed with 5 g of stearyl alcohol, heated to 90° C., and magnetically stirred a...
Embodiment 2
[0035] (1) Dissolve 10mmol of 4-dimethylaminosalicylaldehyde in 10mL of ethanol, add 15mmol of dimethyl malonate and 1mL of catalyst piperidine, stir evenly, and keep stirring the mixture at 70°C for 4h under reflux; Add 50mL deionized water after the reaction finishes, then extract 6 times with ethyl acetate, add 50mL each time, obtain crude product, then separate and purify by chromatographic chromatography, obtain yellow powder chromophore molecule after drying, its 1 H NMR test result is substantially identical with embodiment 1;
[0036] (2) Mix 0.1 g of the purified product in step (1) with 30 g of cetyl alcohol, heat to 80° C., and magnetically stir at a speed of 1500 rpm for 0.5 h, and obtain a yellow solid after the reaction is cooled;
[0037] (3) The yellow solid obtained in 1g step (2) and 5g polymethyl methacrylate are dissolved in 10g methylene chloride, then add 0.05g N-dodecyldimethylamine and 300g deionized water, room temperature After homogenizing for 10 mi...
Embodiment 3
[0041](1) Dissolve 10mmol 4-dimethylaminosalicylaldehyde in 30mL ethanol, add 30mmol dimethyl malonate and 5mL catalyst piperidine, stir evenly, and keep stirring the mixture at 95°C for 1h under reflux; Add 50mL deionized water after reaction finishes, then extract 4 times with ethyl acetate, add 50mL each time, obtain crude product, then separate and purify by chromatographic chromatography, obtain yellow powder chromophore molecule after drying, its 1 H NMR test result is substantially identical with embodiment 1;
[0042] (2) Mix 0.1 g of the purified product in step (1) with 10 g of myristyl alcohol, heat to 60° C., and magnetically stir at a speed of 100 rpm for 2 h, and obtain a yellow solid after the reaction is cooled;
[0043] (3) 1g of the yellow solid obtained in step (2) and 3g of polysulfone were dissolved in 20g of methylene chloride, followed by adding 0.005g of sorbitan monooleate and 150g of deionized water, at room temperature, at 25000rpm After 60 minutes ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com