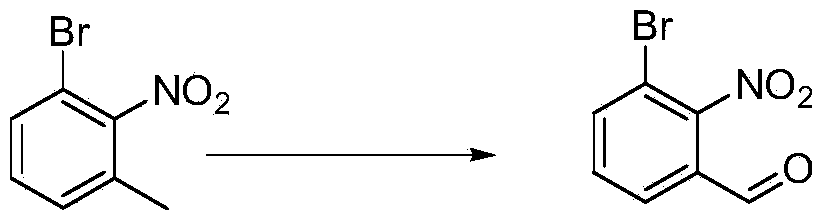
Chemical synthesis method of 3-bromo-2-nitrobenzaldehyde
A technology for the chemical synthesis of nitrobenzaldehyde, which is applied in chemical instruments and methods, organic chemistry, and the preparation of organic compounds. It can solve problems such as low yield and difficult separation, and achieve high yield, low price, and high-efficiency synthesis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
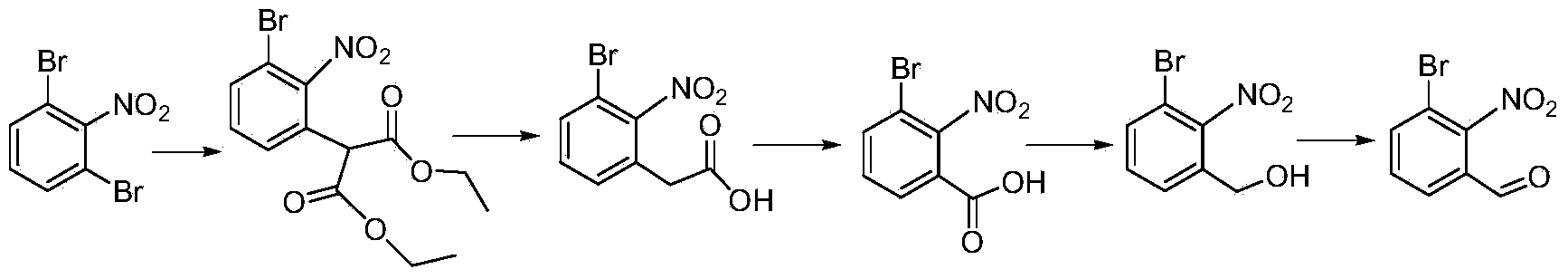
[0025] Synthesis of 2-(3-bromo-2-nitrobenzaldehyde)-dimethyl malonate:
[0026] Add 150mL of N,N-dimethylformamide into the three-necked flask, add 30.0g of 1,3-dibromo-2-nitrobenzene and 28.0g of dimethyl malonate to dissolve, and add 15.0g of sodium carbonate. The temperature of the system was raised to 60°C, and the reaction was detected by thin-layer chromatography to the end. Add 1L of water, extract with ethyl acetate (100mL×3), combine the organic phases, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate to obtain 2-(3-bromo-2-nitrobenzaldehyde)-malonic acid Dimethyl ester 37.1g (96.5%).
[0027] Synthesis of 2-(3-bromo-2-nitrobenzaldehyde)-acetic acid:
[0028] Add 250 mL of acetone to the three-neck flask, add 36.0 g of dimethyl 2-(3-bromo-2-nitrobenzaldehyde)-malonate to dissolve, and add 25 mL of 8M hydrochloric acid. Heated to reflux and reacted, and the reaction was detected by thin layer chromatography until the end...
Embodiment 2
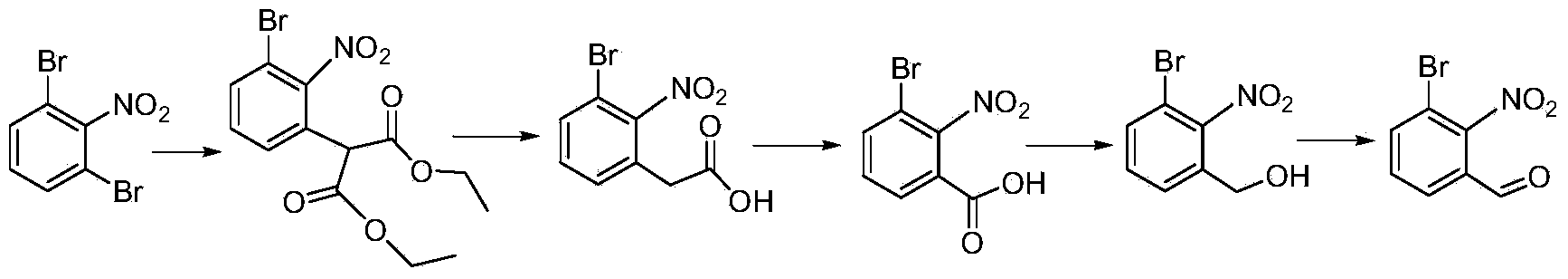
[0036] Synthesis of 2-(3-bromo-2-nitrobenzaldehyde)-dimethyl malonate:
[0037] Add 150 mL of dimethyl sulfoxide to the three-neck flask, add 30.0 g of 1,3-dibromo-2-nitrobenzene and 28.0 g of dimethyl malonate to dissolve, and add 15.0 g of sodium carbonate. The temperature of the system was raised to 50°C, and the reaction was detected by thin-layer chromatography to the end. Add 1L of water, extract with ethyl acetate (100mL×3), combine the organic phases, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate to obtain 2-(3-bromo-2-nitrobenzaldehyde)-malonic acid Dimethyl ester 37.8g (98.3%).
[0038] Synthesis of 2-(3-bromo-2-nitrobenzaldehyde)-acetic acid:
[0039] Add 250 mL of tetrahydrofuran into the three-necked flask, add 36.0 g of 2-(3-bromo-2-nitrobenzaldehyde)-malonate dimethyl ester to dissolve, and add 25 mL of 6M hydrochloric acid. Heated to reflux and reacted, and the reaction was detected by thin layer chromatograph...
Embodiment 3
[0047] Synthesis of 2-(3-bromo-2-nitrobenzaldehyde)-dimethyl malonate:
[0048] Add 150mL of N,N-dimethylformamide into the three-necked flask, add 30.0g of 1,3-dibromo-2-nitrobenzene and 28.0g of dimethyl malonate to dissolve, and add 18.0g of potassium carbonate. The temperature of the system was raised to 60°C, and the reaction was detected by thin-layer chromatography to the end. Add 1L of water, extract with ethyl acetate (100mL×3), combine the organic phases, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate to obtain 2-(3-bromo-2-nitrobenzaldehyde)-malonic acid Dimethyl ester 37.4g (97.3%).
[0049] Synthesis of 2-(3-bromo-2-nitrobenzaldehyde)-acetic acid:
[0050]Add 250 mL of acetone to the three-neck flask, add 36.0 g of dimethyl 2-(3-bromo-2-nitrobenzaldehyde)-malonate to dissolve, and add 25 mL of 10M hydrochloric acid. Heated to reflux and reacted, and the reaction was detected by thin layer chromatography until the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com