Method for synthesizing 6-chlorhydroxyl indole
A technology of looxindole and its synthesis method, which is applied in the direction of organic chemistry, can solve the problems of no patent publications and process characteristics that cannot meet industrial production, so as to improve safety and operation controllability, and reduce post-processing procedures , mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
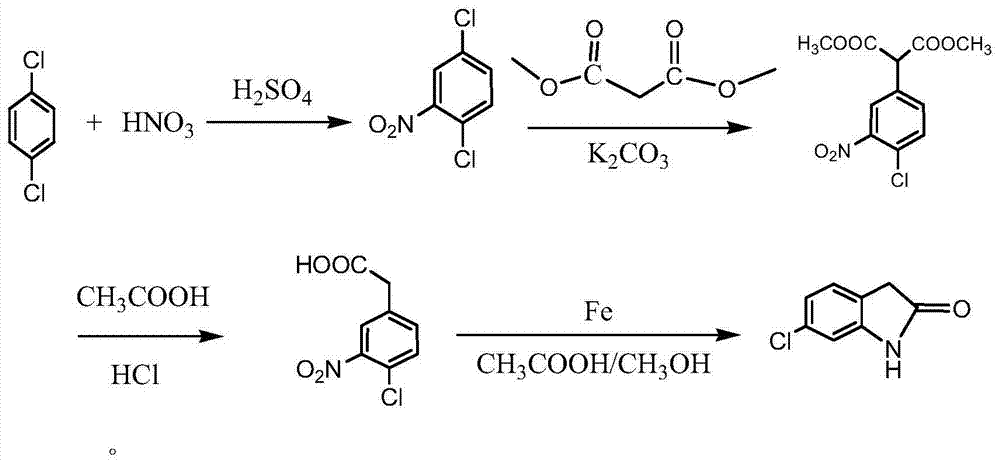
[0035] A kind of synthetic method of 6-chlorooxindole, the steps are as follows:
[0036] (1) Preparation of 2,5-dichloronitrobenzene
[0037] Add 157ml of concentrated sulfuric acid into a 1000ml three-necked bottle, add 196g of p-dichlorobenzene under stirring, add dropwise a mixed acid prepared from 60ml of concentrated sulfuric acid and 75ml of nitric acid under water cooling, after the dropwise addition, slowly heat up to 80-100°C, and keep warm for 2- After 4 hours, after the reaction, cool down to about 60°C, add 392g of cold water, stir for 1 hour, and suction filter to obtain a yellow solid. Melt the yellow solid with 249.5g of hot water at 80°C, stir and wash until neutral, and cool to 15°C , the yellow solid was obtained by suction filtration, that is, 2,5-dichloronitrobenzene.
[0038] Dry 244g, content: greater than 99%, yield 95%.
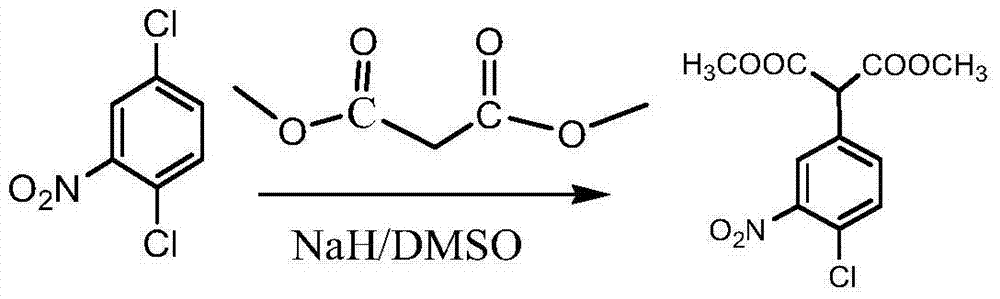
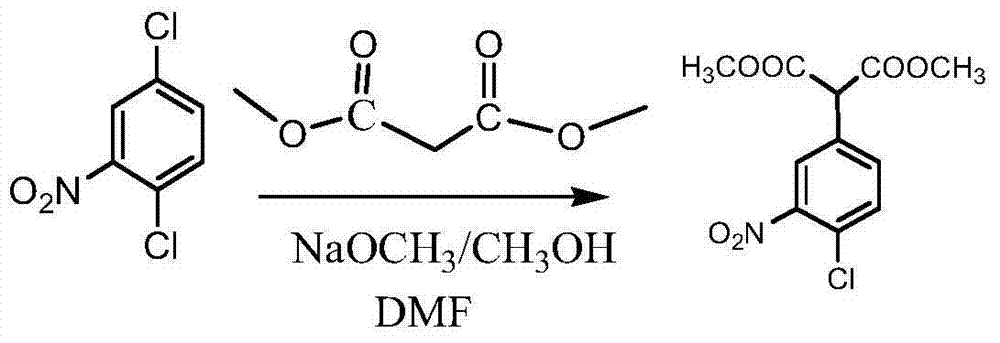
[0039] (2) Preparation of 2-(4-chloro-2-nitrophenyl) dimethyl malonate
[0040] Add 3000ml dimethyl sulfoxide and 280g dimethyl m...
Embodiment 2
[0046] A kind of synthetic method of 6-chlorooxindole, the steps are as follows:
[0047] (1) Preparation of 2,5-dichloronitrobenzene
[0048] Add 156.7ml of concentrated sulfuric acid into a 1000ml three-necked flask, add 196g of p-dichlorobenzene under stirring, and dropwise add a mixed acid prepared by 58.8ml of concentrated sulfuric acid and 70ml of fuming nitric acid under water cooling. -95°C, heat preservation reaction for 2 hours, after the reaction, cool down to about 60°C, add 392g of cold water, stir for 1 hour, and suction filter to get a yellow solid, melt the yellow solid with 257g of hot water at 80°C, stir and wash until neutral , cooled to 15°C, suction filtered to obtain a yellow solid, namely 2,5-dichloronitrobenzene. Dry 251.2g, content: 99.7%, yield 97.6%.
[0049] (2) Preparation of 2-(4-chloro-2-nitrophenyl) dimethyl malonate
[0050] Add 3000ml dimethyl sulfoxide and 326g dimethyl malonate into a 5000ml three-necked flask, add 251.2g of 2,5-dichloron...
Embodiment 3
[0056] A kind of synthetic method of 6-chlorooxindole, the steps are as follows:
[0057] (1) Preparation of 2,5-dichloronitrobenzene
[0058] Add 178ml of concentrated sulfuric acid into a 1000ml three-necked bottle, add 196g of p-dichlorobenzene under stirring, add dropwise a mixed acid prepared from 68ml of concentrated sulfuric acid and 95ml of nitric acid under water cooling, after the dropwise addition, slowly heat up to 80-100°C, and keep warm for 2- After 4 hours, cool down to about 60°C after the reaction, add 450g of cold water, stir for 1 hour, and filter to obtain a yellow solid. Melt the yellow solid with 250g of hot water at 80°C, stir and wash until neutral, and cool to 15°C , Suction filtration to obtain a yellow solid, namely 2,5-dichloronitrobenzene. Dry 239g, content: greater than 99%, yield 92.9%.
[0059] (2) Preparation of 2-(4-chloro-2-nitrophenyl) dimethyl malonate
[0060] Add 3000ml dimethyl sulfoxide and 370g dimethyl malonate into a 5000ml three-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com